Abstract
Background: Long-term survival rates for most types of childhood cancers have improved dramatically over the past decades. However, because of advances in multimodality treatments, cancer survivors nowadays more often face long-term complications, including diminished gonadal and reproductive function. The aim of this study was to identify whether the use of fertility treatments among early onset (0–34 years) cancer survivors giving birth differed from that among siblings giving birth and to identify the subgroups of cancer survivors that were most likely to require fertility treatments.
Material and methods: Nationwide cancer and birth registries were merged to identify 1974 post-diagnosis deliveries of cancer survivors and 6107 deliveries of female siblings in 2004–2013. Unconditional multivariate logistic regression models were used to estimate the risk for different fertility treatments namely assisted reproductive technology, intrauterine insemination and ovulation induction. We adjusted for maternal age, year of delivery, parity and smoking.
Results: We found overall significantly increased odds for use of any fertility treatments in survivors compared to siblings (OR 1.84, 95% CI 1.18–2.86). As time from cancer treatment increased, the odds for need of fertility treatments increased, being highest at 11 to 15 years post cancer treatment (OR 2.88, 95% CI 1.13–7.30). Survivors diagnosed at ages 25–34 years had the highest odds for use of fertility treatments compared to siblings (OR 2.31, 95% CI 1.01–5.32).
Conclusions: Our study supports previous findings indicating that cancer survivors have an increased risk for subfertility. Survivors diagnosed in their childhood had the lowest risk for fertility treatment and seemed to get pregnant with less extensive fertility treatments than survivors diagnosed as adults. Time elapsed from cancer treatment played a central role, increasing the need for fertility treatments compared to siblings, suggesting that cancer therapies might lead to diminished ovarian reserve.
Introduction
Long-term survival rates for most types of childhood cancers have improved dramatically over the past decades. In a recent study the 5-year survival of Finnish children diagnosed with cancer in 2000–2010, was as high as 81% in the most recent period [Citation1], similar to published European figures [Citation2]. Because of advances in multimodality treatments, cancer survivors nowadays more often face long-term complications, including diminished gonadal and reproductive function. The relative probability of parenthood in former early onset cancer patients has been reported to be reduced even by up to 50% compared to their siblings [Citation3]. Furthermore, fertility has been reported to be a major long-term concern among cancer survivors [Citation4]. A recent study showed that female cancer survivors experiencing reproductive concerns are more likely to experience moderate or even severe depression [Citation5].
Prevalence of infertility, estimated to be approximately 10–15% of the Western population, is increasing worldwide. One of the reasons might be the use of gonadotoxic oncologic treatment [Citation6]. In Finland, 13–17% of women have reported difficulties in trying to conceive within 12 months at some point in their lives [Citation7]. There are, however, limited published data on infertility among cancer survivors and even less on use of fertility treatments.
The aim of this study was to identify whether the use of fertility treatments among early onset (0–34 years) cancer survivors giving birth differed from that among siblings giving birth and to identify the subgroups of cancer survivors that were most likely to require fertility treatments.
Material and methods
Each individual living in Finland is given a personal identity number (PIN) which enables linkage between different registries and databases. This retrospective, nationwide, population based register study used data derived from linkage between the Finnish Cancer Register, the Central Population Register and the Medical Birth Register.
Registers
The population-based Finnish Cancer Register (FCR) began registration in 1953 with high coverage for solid tumors (close to 100%) and 92% for hematologic malignancies [Citation8]. Data in the register include details of primary cancer including anatomical site, histology, staging, primary cancer treatment and time of cancer diagnosis.
The Central Population Register (CPR), founded in 1969, covers all Finnish residents and includes data on residential history and emigration, as well as date of death. Individuals born in 1955 or thereafter can be linked reliably to their parents, siblings and offspring.
The Medical Birth Register (MBR) contains data on all mothers giving birth and their children born in Finland from 1987 onwards. All live births and stillbirths with a birth weight of more than 500g or a pregnancy continuing for at least 22 weeks are registered. Data on less than 0.1% of infants are missing in the MBR [Citation9]. Data on assisted reproductive technology (ART) (including in vitro fertilization, intracytoplasmic sperm injection and frozen embryo transfer) have been collected since October 1990 and from January 2004 onwards data on also intrauterine inseminations and ovulation inductions are registered.
Study population
shows the flow chart of the study population, initially consisting of 13,799 female cancer patients (hereafter referred to as survivors) diagnosed with a malignant neoplasm or benign brain tumor and their 21,640 female full- and half-siblings.
Figure 1. Female cancer survivors and their female siblings. Criteria for inclusion of births and number of mothers and births included in the final analyses.
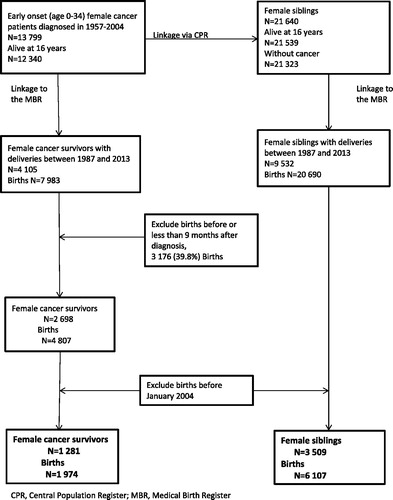
By linkage to the MBR, we identified 4105 survivors and 9532 siblings, with pregnancies between January 1987 and December 2013. After excluding women whose cancer was diagnosed during pregnancy or who had given birth prior to being diagnosed with cancer we were left with 2698 survivors.
In this study we compared use of different types of fertility treatments (available since January 2004) among cancer survivors and siblings during the most recent years. After further excluding survivors and siblings giving birth before January 2004, we were left with 1281 survivors with 1974 pregnancies and 3509 siblings with 6107 pregnancies (). Of these 1281 survivors and 3509 siblings, 474 (37.0%) survivors and 1382 (39.4%) siblings had given birth prior to 2004.
Statistical analyses
Unconditional univariate and multivariate logistic regression analysis was used to estimate odd ratios (ORs) with 95% confidence intervals (CI) for the dichotomous outcomes comparing survivors to siblings. As multiple pregnancies of the same mother were included in the analyses, random effects modeling was used to take into account the dependent nature of the data of children born to the same mother. In the multivariate analysis we considered maternal age at delivery, calendar time period of delivery, maternal smoking and parity as possible confounders.
The fertility treatments were sub-classified into three different groups: ART, intrauterine insemination and ovulation induction which were also included in the variable any fertility treatment.
We also performed multivariate sub-analyses stratifying by age at diagnosis (0–14, 15–24 and 25–34 years), time from diagnosis to delivery (0–5, 6–10, 11–15, 16–25 and 26–34 years) and treatment (chemotherapy, radiotherapy and surgery only). In these analyses, 95% CIs were corrected for multiple comparisons using the Bonferroni correction.
Calendar time was categorized into two time periods (2004–2008 and 2009–2013), maternal age into four groups (younger than 25, 25–29, 30–34 and 35 years or older) and parity into four groups (null, one, two or more and missing). Information on maternal smoking was available as: smokers, nonsmokers, and those with missing information.
For descriptive purposes, the difference in categorical variables between survivors and siblings was analyzed using the χ2-test (). All analyses were conducted using STATA version 12.1 (StataCorp, College Station, TX, USA).
This study protocol, including the use of administrative health data, was approved by the THL National Institute for Health and Welfare (Dnr THL/1/5.05.00/2014) and includes an ethical evaluation of the study.
Results
As seen in , the majority of the survivors (70%) were diagnosed with cancer between 1993 and 2004. More than 72% were 15 years or older at cancer diagnosis. The most common diagnostic group was carcinomas and other malignant epithelial neoplasms (41%), followed by lymphomas (19%) and leukemia (12%).
Table 1. Diagnostic characteristics of female cancer survivors with post-diagnosis births between 2004 and 2013.
Table 2. Descriptive characteristics of post-diagnosis births of female cancer survivors and births of female siblings between 2004 and 2013 as well as p-values.
The age at delivery was higher among survivors than among siblings (p-value < .001); furthermore, survivors were more likely to be nulliparous (p-value < .001). There were no differences in the time period (p-value .069) or maternal smoking (p-value .975) between the survivors and siblings ().
Altogether, 103 out of 1974 births (5.2%) of survivors and 170 out of 6107 births (2.8%) of siblings began after fertility treatments (OR 1.84, 95% CI 1.18–2.86). The difference by type of fertility treatment did not reach statistical significance. Some women had undergone several different treatments when getting pregnant ().
Table 3. Unadjusted and adjusted odd ratios for fertility treatments between 2004 and 2013 among women with a history of cancer compared with female siblings.
When it came to age at cancer diagnosis (Supplemental Table 1), survivors diagnosed as adults (aged 25–34 years) had the highest odds for use of fertility treatments (7.7%) compared to siblings aged 25 years or older (3.2%) when giving birth (OR 2.31, 95% CI 1.01–5.32). Survivors diagnosed as adults also had the highest odds for ART treatments (OR 3.13, 95% CI 1.03–9.52). On the contrary, cancer survivors diagnosed in childhood (aged 0–14 years) had the lowest odds for use of overall fertility treatments (3.7%) compared to siblings (OR 1.56, 95% CI 0.68–3.61) but had significantly increased odds for intrauterine inseminations (OR 3.42, 95% CI 1.08–10.82).
Looking at time from cancer diagnosis to delivery (Supplemental Table 2), we observed that the odds for fertility treatments increased over time, being at its lowest (OR 0.75, 95% CI 0.26–2.15) less than six years from treatment and highest (OR 2.88, 95% CI 1.13–7.30) 11–15 years from cancer treatments with no statistically significant increase in odds thereafter.
In analyses stratified by cancer treatments (Supplemental Table 3), it was shown that 36 out of 633 births of survivors (5.7%) receiving radiotherapy and 170 out of 6107 births of siblings (2.8%) began after fertility treatments (OR 2.24, 95% CI 1.01–4.96). However, when excluding 160 patients with radioactive iodine use, the use of fertility treatments was not significantly higher in this group (OR 1.49, 95% CI 0.56–3.99). A separate analysis of survivors treated with abdominal radiotherapy did not show increased odds for use of any fertility treatments but the numbers were small: only 4 out of 92 survivors treated with abdominal radiotherapy received fertility treatments. Survivors treated with surgery only or chemotherapy did not have significantly increased odds for fertility treatments.
Discussion
Female cancer survivors giving birth have an almost two-fold higher use of fertility treatments compared to their siblings. Among survivors delivering, those diagnosed in their childhood had the lowest risk for use of fertility treatment and they seemed to have less need for more advanced fertility treatments than survivors diagnosed as adults. Time elapsed from cancer treatment played a central role, with increasing use of fertility treatments over time, being highest at 11–15 years after cancer treatments.
The main strengths of this study include population-based data originating from consistent, homogenous and reliable registers covering a large number of births. In addition, the information on all cancer cases diagnosed since birth was retrieved and the MBR data was extracted similarly for survivors and siblings. Our data allowed follow-up for outcomes which extended into very recent years. This is critical since fertility treatments have become more prevalent and widespread in clinical practice in the last decades [Citation10]. We also had information on important confounders to adjust for different factors that might interfere with the outcome.
The main limitation of our study is that it measures the use of fertility treatments only among survivors and siblings giving birth. Also information on the indication for fertility treatments or whether the ART was carried out using autologous or donor oocytes was not available in the MBR. Further studies, including cancer survivors with unsuccessful fertility treatments, should be considered. A previous study showed that all fertility treatments are not documented in the MBR [Citation9] as this information relies on self-report of the mother. We suspect that the true incidence of fertility treatments leading to pregnancy in our cohort is even higher than evident based on the MBR data. However, this possible underestimation applies similarly to survivors and siblings and as we report odds ratios, we consider our results valid.
Another limitation is the lack of information on detailed therapeutic exposures (such as radiation doses, field location and specific chemotherapeutic agents).
There are only a few studies focusing on subfertility among early onset female cancer survivors. The largest of them, the Childhood Cancer Survivor Study [Citation11], reported a 48% increased risk for clinical infertility (defined as the inability to conceive after one year or more of unprotected intercourse during the fertile phase of the menstrual cycle). Increasing doses of uterine radiation and chemotherapy with alkylating agents increased the risk of infertility in a dose-dependent fashion. Interestingly they found that cancer survivors were equally likely to seek treatment for infertility comparing to their siblings but they were less likely to be prescribed drugs for treatment of infertility. This finding is in conflict to our results where survivors had more fertility treatments than siblings.
We found one study [Citation12] that, similar to ours, analyzed the risk of fertility treatments in former cancer survivors giving birth based on registry data. They also found an almost two-fold risk for fertility treatments in cancer survivors compared to females without a cancer history. Comparison pregnancies were matched by maternal age-group, parity and year of delivery. This study, however, did not classify different types of fertility treatments and did not take into account different cancer treatments or elapsed time from cancer treatment. Also, they included only survivors diagnosed with cancer at the age of 15 to 39 years.
Another recent study [Citation13] found the likelihood of a live birth after ART among female cancer survivors using autologous oocytes to be reduced compared to healthy women treated with ART. However, the likelihood was similar when donor oocytes were used. In our study, time elapsed from cancer treatment appeared to increase the need for fertility treatments supporting previous findings that cancer therapies might lead to diminished ovarian reserves resulting in a narrowed fertile time window.
Collaboration between oncologists and reproductive medicine providers is important. Our data may be applicable in the clinical setting for those treating cancer patients, especially when identifying patients with exceptionally high risk for infertility and for providing timely interventions. Fertility preservation programs are rapidly being developed. In the light of current knowledge, with the uncertainty in determining the actual risk for infertility, counseling should be offered to all young cancer patients. Furthermore, fertility treatments should be available for all the cancer survivors.
IONC_1304653_Supplemental_appendix__1_.docx
Download MS Word (18.1 KB)Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Madanat-Harjuoja LM, Pokhrel A, Kivivuori SM, et al. Childhood cancer survival in Finland (1953-2010): a nation-wide population-based study. Int J Cancer. 2014;135:2129–2134.
- Gatta G, Botta L, Rossi S, et al. Childhood cancer survival in Europe 1999–2007: results of EUROCARE-5 ? a population-based study. Lancet Oncol. 2014;15:35–47.
- Madanat LM, Malila N, Dyba T, et al. Probability of parenthood after early onset cancer: a population-based study. Int J Cancer. 2008;123:2891–2898.
- Peate M, Meiser B, Hickey M, et al. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116:215–223.
- Gorman JR, Su I, Roberts SC, et al. Experiencing reproductive concerns as a female cancer survivor is associated with depression. Cancer. 2015;121:935–942.
- Petraglia F, Serour GI, Chapron C. The changing prevalence of infertility. Int J Gynaecol Obstet. 2013;123(Suppl 2):S4–S8.
- Klemetti R, Raitanen J, Sihvo S, et al. Infertility, mental disorders and well-being ? a nationwide survey. Acta Obstet Gynecol Scand. 2010;89:677–682.
- Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol. 1994;33:365–369.
- Gissler M, Klemetti R, Sevón T, et al. Monitoring of IVF birth outcomes in Finland: a data quality study. BMC Med Inform Decis Mak. 2004;4:3.
- Terävä A, Gissler M, Hemminki E, et al. Infertility and the use of infertility treatments in Finland: prevalence and socio-demographic determinants 1992–2004. Eur J Obstet Gynecol Reprod Biol. 2008;136:61–66.
- Barton S, Najita J, Ginsburg E, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013;14:873–881.
- Haggar FA, Pereira G, Preen D, et al. Adverse obstetric and perinatal outcomes following treatment of adolescent and young adult cancer: a population-based cohort study. PLoS One. 2014;9:e113292.
- Luke B, Brown M, Missmer S, et al. Assisted reproductive technology use and outcomes among women with a history of cancer. Hum Reprod. 2016;31:183–189.
