Abstract
Background: Oral carcinomas (OCs) make up a significant proportion of head and neck carcinomas (HNCs) and are an important cause of morbidity and mortality globally. The purpose of this population-based study was to determine trends in incidence and survival in OC in the Danish population from 1980 to 2014.
Material and methods: This study covered all patients registered in the nationwide Danish cancer registry (DCR) in the period 1980–2014. Age-adjusted incidence rate (AAIR) per 100,000 and annual percentage change (APC) were evaluated. Also, 5-year overall survival (OS) was calculated with Cox regression analysis in relation to location, gender, age, and calendar year at diagnosis.
Results: Altogether, 8299 patients with oral cancer were identified, 5062 (61%) of whom were males and 3237 (39%) were females. The median age at diagnosis was 63 years. The AAIR of patients with OC increased from 1.9 per 100,000 in 1980 to 3.5 per 100,000 in 2014, and we observed a significant increase in 5-year OS of 12% points (a relative increase of 38%) from the period 1980–1984 to 2005–2009. Women were found to have a better prognosis than men.
Conclusions: We found an unexpected increase in the age-standardized incidence of OC during the last 30 years in Denmark, and also an improvement in survival. The 5-year OS was significantly better in recent years even when we adjusted the analysis for relevant covariates.
Introduction
Globally, the annual incidence of oral carcinomas (OCs) is approximately 300,000 [Citation1]. OC makes up a significant proportion of head and neck cancer, and is an increasing and important cause of morbidity and mortality worldwide [Citation1–3]. The vast majority of cancers of the oral cavity is derived from the multi-layered squamous epithelium and is referred to as oral squamous cell carcinoma (OSCC). Tobacco and alcohol consumption are the major risk factors for the development of OC, and these are known to have a synergistic effect [Citation2,Citation4]. The preferred treatment for localized OCs (i.e., at stages I and II) is surgery, and for advanced OC in general, both surgery and chemoradiotherapy are recommended [Citation5,Citation6]. As for all head and neck carcinomas, survival with OC is closely related to the stage at presentation; reports have shown that the overall survival (OS) in patients with stage-I OC approaches 83%, while for patients diagnosed with stage IVB; the OC is approximately 34%. Cervical lymph node metastasis is known to be the single most important prognostic factor, with the overall 5-year survival dropping from 82 to 53% in OC patients with cervical lymph node involvement [Citation7,Citation8].
In other studies, the incidence of OC is reported to be increasing in several European countries but decreasing in the USA and Canada [Citation3]. Despite recent clinical improvements in diagnosis and treatment, the 5-year survival rate in patients with OC has been reported to have remained stable at approximately 50% [Citation2,Citation5,Citation6]. The evaluation of OC incidence has often been hindered by the inclusion of non-oral subsites, such as oropharyngeal cancer and lip cancer. There has been no consistent, stringent report of patients with only cancer of the oral cavity, demarcated anteriorly by the vermillion junction of the lips and posteriorly by the junction of the hard and soft palate, and to the line of the circumvallate papillae on the dorsal tongue.
Since 1968, the central population register (CPR) has issued all Danish residents with a unique personal identification number (the CPR number), which is used in all national registries and enables accurate linkage of data on one individual. The CPR has updated information on vital status, e.g., date of death and date of emigration [Citation9].
The Danish cancer registry (DCR) covers the entire Danish population and has been registering the incidence and prevalence of cancer since 1943. This affords an excellent opportunity to follow population-level trends in OC incidence and survival, reporting of data has been mandatory since 1987 [Citation10].
The CPR and the DCR offer unique possibilities for carrying out nationwide epidemiological studies. The aim of this registry-based study was, therefore, to determine the changing trends in OC from a nationwide point of view during a long period of follow-up, and to evaluate changes in incidence and overall survival.
Material and methods
In Denmark, head and neck cancer (HNC) is treated at highly specialized university centers based on a multidisciplinary collaboration between head and neck surgeons, oncologists, hospital dentists, pathologists, radiologists and specialists in nuclear medicine. The Danish population is provided with universal, tax-supported access to general practitioners and hospitals through the Danish healthcare system. The CPR number is used in all national registries and enables accurate linkage of individual data [Citation9]. This study was conducted using a source population from the DCR.
During the study period (1980–2014), two different versions of the International Classification of Diseases (ICD) were used. The ICD-7 codes used until 2003 were converted to ICD-10 codes. All patients registered with oral cavity cancers in Denmark from 1980 to 2014 with the following ICD-10 codes were included from the DCR: for tongue cancers, DC02–DC029 excluding lingual tonsils DC024; for gingival cancers, DC03–DC039; for floor of the mouth (FOM) cancers, DC04–DC049, and for cancers of the hard palate, DC050 and DC059. We also included an unspecified category of oral cancers with the following ICD-10 codes: DC06–DC069. We excluded the base of tongue cancers with ICD-10 codes: DC01–DC019, since these are considered to be oropharyngeal cancer.
Data on age were derived from the DCR; vital status and dates of emigration and immigration were obtained separately from the CPR. Age-specific population counts were taken from the National Statistical Database [Citation9]. The study was approved by the Danish Data Protection Agency (2012-58-0004).
Statistical analyses
Statistical analysis was done using IBM SPSS 22 (IBM, SPSS, Chicago, IL, USA) and R Statistics version 3.2.2 (Stanford University, Stanford, CA, USA). The average annual percentage change (AAPC) in the incidence was calculated using Joinpoint Trend Analysis software version 4.2.0.2 (National Cancer Institute, Bethesda, MD, USA) with growth assumed to be logarithmic and to follow the formula ln(y) = xb. The joinpoint regression analysis estimates possible joinpoints (significant change in trend, i.e., ‘trend breaks’) with either a straight line or a segmented line if there are trend breaks. We allowed a maximum of one joinpoint for the analysis from 2004 to 2014 and a maximum of five joinpoints in the analysis of AAPC from 1980 to 2014. We calculated 5-year age-specific crude incidence rates and age-standardized incidence rates per 100,000 using the direct method with R Statistics and the EpiTools package using the WHO World Standard Population as reference [Citation11]. The endpoint in the survival analysis was OS. OS was defined as the time from diagnosis to death from any cause. Patients who were alive on the last date of follow-up were censored at this date. Our cohort was divided into 5-year intervals from 1980 and onwards and we used Kaplan–Meier curves or Cox proportional hazard models to illustrate survival differences, with log-rank tests to test for significant differences in survival. We considered p values of < .05 to indicate statistical significance.
Results
Altogether, 8299 patients with oral cancer were identified between 1980 and 2014 (), with a median age of 63 years at diagnosis. Of these, 5062 (61%) were males and 3237 (39%) were females.
Table 1. Univariate analysis of prognostic factors for overall survivalTable Footnotea.
Trends of incidence of oral cancer in Denmark from 1980 to 2014
For men, the AAI rate per 100,000 increased from 4.2 in 1980 to 7.2 in 2014 (, ). We observed a trend break in the incidence, where the increase in incidence leveled off from 1996 and the APC changed from 3.3 to 0.68% (). For women, the incidence increased from 1.5 to 2.3 in the same period () with an AAPC of 1.8% (). The total number of patients with oral cancer increased from 136 in 1980 to 326 in 2014, converted to the age-adjusted incidence it increased from 1.9 per 100,000 in 1980 to 3.5 per 100,000 in 2014 (). We observed a trend break from 1996 and a change in APC from 3.5 to 0.86% (). Regarding location, the incidence of tongue cancers increased in the entire period, while the incidence of FOM tumors and palate tumors decreased after trend breaks in 1997 and 1994 ().
Figure 1. Trends in age-adjusted incidence rates per 100,000 for oral cancer, according to sex (panel A), anatomical location (B), 5-year age group for men and women (C) and site-specific incidence rates (D) based on 5-year age groups. The incidence rates are based on the mean incidence from 1980 to 2014. FOM: floor of the mouth.
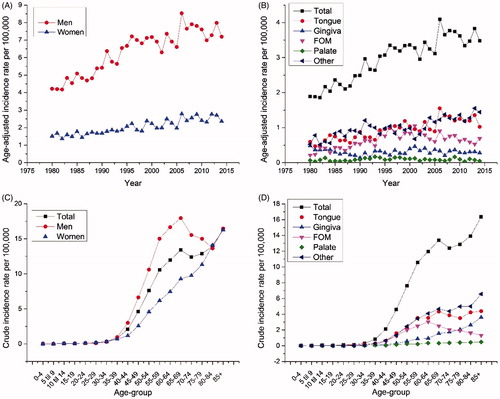
Table 2. Joinpoint regression analysis demonstrating trend breaks in the age-standardized incidence rates.
The incidence rates of oral cancers peaked at ages 65–69 years, and after a small decline they peaked again after 80 years of age in both men and women (). The same general observation with a peak incidence at the age of 65–69 years and after 80 years was observed for all oral subsites, except for FOM, where there was no peak after 65–69 years ().
Increased 5-year survival rate from 1980 to 2014
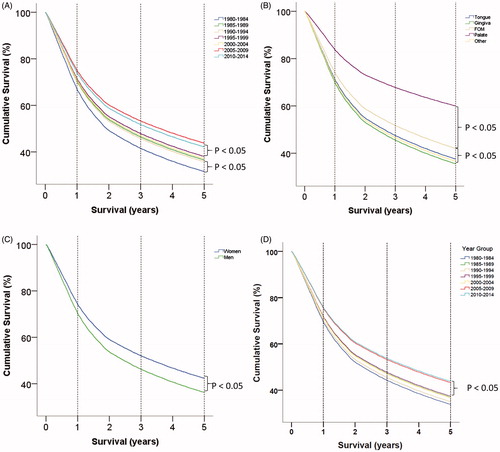
Altogether, 6403 patients died during the study period, and they had a median follow-up of 2.5 years (range 0–36 years). The 5-year OS rate was seen to increase significantly from the period 1980–1984 to 1985–1989 (). A significant increase in survival was also observed from the period 1995–1999 to 2005–2009 (). In particular, the 1-, 3- and 5-year survival rates increased from 66, 40 and 32%, respectively, in the period 1980–1984 to 72, 45 and 36% in 1985–1989. This again increased significantly to the period 2005–2009, with corresponding survival rates of 74, 54 and 44%. This was an overall increase of 12% points, representing a relative increase in the 5-year OS rate of 38% from 1980–1984 to 2005–2009.
Figure 2. Cox regression survival curves. A. Comparison of 5-year overall survival based on 5-year age groups, where the variable is coded as a repeated variable. Notice that there are two periods where an increase in survival is observed. B. Comparison of 5-year overall survival based on oral subsite. C. Comparison of 5-year overall survival based on gender. D. Cox regression survival curve.
Factors associated with poor survival in oral cancer
We investigated whether specific anatomical locations in the oral cavity might confer a poor prognosis, and found that patients with an OC located in either the FOM or the hard palate had a significantly better prognosis than those with an OC at other anatomical locations (, ). We also found that there was significantly better survival in women than in men, with 5-year survival rates of 43 and 36%, respectively ().
Finally, using a multivariate Cox regression analysis, we also examined whether the above findings regarding increasing survival in different time periods were independent of gender and age, and the increasing survival remained significant for the comparison between 2000–2004 and 2005–2009 (, ).
Table 3. Multivariate analysis of independent factors influencing 5-year overall survivalTable Footnotea.
Discussion
This is the hitherto largest nationwide registry-based study to investigate the incidence of and survival rates for, OC in Denmark. Interestingly, we found that both the incidence and the survival have increased over the past 34 years. A great strength of this study is that registration of patient data in the DCR is mandatory and that the healthcare system in Denmark provides all patients with an equivalent standard of evidence-based treatment.
During the study period (1980–2014), the age-adjusted incidence rate (AAIR) for OC increased from 1.9 to 3.5 cases per 100,000 Danish inhabitants. Interestingly, we observed a trend break in OC incidence from 1996, where the APC was only 0.86% (as compared to an APC of 3.5% in the previous period), suggesting that the peak in incidence of OC in Denmark may soon be apparent. The APC was equally distributed between men and women, but with a markedly higher incidence observed in men. It is interesting that the increase in incidence was still significant even though the incidence rates were age-standardized―and, therefore, did not represent an increase due to aging of the population. The rising incidence of OC parallels findings from other European countries, such as Finland, Estonia and England. In contrast, the incidence of OC in the USA and Canada has been reported to be stable or decreasing [Citation3]. An isolated increase in tongue cancer has been described in other European countries and in the USA [Citation12], and in this study it was also one of the subsites with the highest increase. We have considered cancer in the lingual tonsil and the base of the tongue to be oropharyngeal cancers, so we cannot compare our results fully with other studies [Citation3].
The known major risk factors for OSCC are tobacco use and excessive alcohol intake. Alcohol consumption in Denmark rose up to the early 1980s [Citation13], and while alcohol consumption per capita has decreased during the past two decades in Denmark, we do not know how the decreasing overall alcohol consumption has affected the proportion of heavy drinkers [Citation14]. Tobacco consumption has decreased in Denmark since the 1970s, but the number of heavy smokers has remained stable in men and increased in women [Citation15]. Lung cancer is another smoking-related cancer and the incidence of lung cancer in most age groups peaked for men in 2010, whereas the incidence in women has not yet peaked [Citation13]. The increasing incidence in OC may be explained by the stable number of heavy smokers and the high alcohol consumption, since heavy smokers and heavy drinkers have a 35 times the risk compared to abstainers [Citation16].
While human papillomavirus (HPV) has previously been implicated as a risk factor for oral cancer, recent studies have suggested that, in comparison to oropharyngeal cancers, the importance of HPV in the development of OC is small to non-existent [Citation17].
An important finding in this study was the increased 5-year OS from OC from 1980 to 2014. Many previous studies have reported that the OS rate for OC has not changed during the last 30 years [Citation17,Citation18]. However, we found a nominal increase in 5-year OS from 32 to 44% (i.e., a relative increase of 38%) from the beginning of the study period (1980–1984) to 2005–2009. This positive change in survival was still significant when we adjusted for age and gender in a multivariate analysis. The improved survival observed for women in this study has been described previously [Citation2,Citation19].
The increased survival for patients with OC may be explained by improvements in therapeutic management. In 2003, a national Danish guideline for treatment of OC was developed. This guideline was in accordance with international recommendations and was developed to facilitate a uniform treatment policy. The guideline recommended single-modality treatment for stage I, stage II, and some stage III, and combined modality treatment for stages III and IV. Surgery was the preferred treatment when radical excision of the tumor and possible lymph node metastases was possible, with acceptable esthetic and functional outcome [Citation20]. This policy of managing OC with more surgery resulted in better survival [Citation21]. During the study period, better radiation therapy techniques have been introduced, such as moderately accelerated radiotherapy [Citation22], concurrent chemoradiotherapy, improved imaging and highly conformal radiotherapy. Another important factor may have been centralization and development of new surgical procedures, including sentinel node biopsy (SNB), for OC during the period of this study [Citation23].
In 2007, a fast-track program for patients with suspicion of HNC was introduced in Denmark to reduce unnecessary waiting time, a known risk factor for poorer survival in HNC [Citation24]. The implementation, including in particular an increase in the number of linear accelerators in Denmark, successfully reduced the time course from the suspicion of head and neck cancer until treatment from 57 to 29 d [Citation25]. It could be a contributor to the increased survival during this period. A higher awareness in the primary sector and better supportive care may also have contributed.
All our data were anonymized and this resulted in some limitations. We were unable to compare the diagnosis from the DCR to the patient file and we did not have access to the pathological files in order to achieve a higher certainty i.e., the TNM classification. We have found that oral cancer located in the FOM and hard palate has a significantly better prognosis than OC at other anatomical locations. This is inconsistent with the literature [Citation19], and even though we had a large cohort, we cannot conclude that the 2859 patients (34.4%) with unknown OC location (referred to as ‘other’) were evenly distributed between the different anatomical locations.
The TNM classification was not a parameter that we could use in this study. The TNM registration takes place very early in the diagnostic work-up, and many are changed later in the process. In order to use the TNM classifications and have access to the pathological files, we would have to verify the data in the patient notes―which was impossible, due to anonymization of data.
In conclusion, we found an unexpected increase in the age-standardized incidence of OC during the past 30 years, where the APC decreased significantly from 1996. This was concurrent with a simultaneous increase in the 5-year OS, even when adjusted for age and gender. Possible reasons for this improvement may be higher awareness, earlier diagnosis, the ‘fast-track’ approach, and improved imaging and treatment techniques, both surgical and non-surgical.
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN. 2012.
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. Available from: http://dx.doi.org/10.1016/j.oraloncology.2008.06.002
- Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50:387–403.
- Morse DE, Psoter WJ, Cleveland D, et al. Smoking and drinking in relation to oral cancer and oral epithelial dysplasia. Cancer Causes Control. 2007;18:919–929.
- Jerjes W, Upile T, Petrie A, et al. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head Neck Oncol. 2010;2:1–21.
- Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J Clin. 2015;65:401–421.
- Stoeckli SJ, Alkureishi LWT, Ross GL. Sentinel node biopsy for early oral and oropharyngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2009;266:787–793.
- Kreppel M, Eich HT, Kübler A, et al. Prognostic value of the sixth edition of the UICC’s TNM classification and stage grouping for oral cancer. J Surg Oncol. 2010;102:443–449.
- Thygesen LC, Daasnes C, Thaulow I, et al. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39:12–16. Available from: http://sjp.sagepub.com/cgi/doi/10.1177/1403494811399956
- Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39:42–45.
- Ahmad OB, Boschi-Pinto C, Lopez AD. Age standardization of rates: a new WHO standard. GPE Discuss Pap Ser. 2001;1–14. Available from: http://www.who.int/healthinfo/paper31.pdf
- Annertz K, Anderson H, Palmér K, et al. The increase in incidence of cancer of the tongue in the Nordic countries continues into the twenty-first century. Acta Otolaryngol. 2012;132:552–557.
- Kristiansen C, Schytte T, Hansen KH, et al. Trends in lung cancer in elderly in Denmark, 1980–2012. Acta Oncol. 2016;55:46–51.
- Sundhedsstyrelsen. Statens Seruminstitut. Alkoholstatistik 2015b. Nationale data. 2015.
- Osler M, Prescott E, Gottschau A, et al. Trends in smoking prevalence in Danish adults, 1964–1994. The influence of gender, age, and education. Scand J Soc Med. 1998;26:293–298.
- Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287.
- Malik UU, Zarina S, Pennington SR. Oral squamous cell carcinoma: key clinical questions, biomarker discovery, and the role of proteomics. Arch Oral Biol. 2016;63:53–65. Available from: http://dx.doi.org/10.1016/j.archoralbio.2015.11.017
- Ragin CCR, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–1820.
- Listl S, Jansen L, Stenzinger A, et al. Survival of patients with oral cavity cancer in Germany. PLoS One. 2013;8:1–6. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3548847&tool=pmcentrez&rendertype=abstract
- Bilde A, von Buchwald C, Johansen J, et al. The Danish national guidelines for treatment of oral squamous cell carcinoma. Acta Oncol. 2006;45:294–299.
- Charabi B, Torring H, Kirkegaard J, et al. Oral cancer-results of treatment in the Copenhagen University Hospital. Acta Otolaryngol Suppl. 2000;543:246–247.
- Overgaard J, Hansen HS, Specht L, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet. 2003;362:933–940.
- von Buchwald C, Bilde A, Shoaib T, et al. Sentinel node biopsy: the technique and the feasibility in head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2002;64:268–274.
- Murphy CT, Galloway TJ, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. JCO. 2016;34:169–178. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26628469
- Toustrup K, Lambertsen K, Birke-Sorensen H, et al. Reduction in waiting time for diagnosis and treatment of head and neck cancer – a fast track study. Acta Oncol. 2011;50:636–641.
