Abstract
Introduction: Geometrical uncertainties can result in a delivered dose to the tumor different from that estimated in the static treatment plan. The purpose of this project was to investigate the accuracy of the dose calculated to the clinical target volume (CTV) with the dose-shift approximation, in stereotactic body radiation therapy (SBRT) of lung tumors considering setup errors and breathing motion. The dose-shift method was compared with a beam-shift method with dose recalculation.
Material and methods: Included were 10 patients (10 tumors) selected to represent a variety of SBRT-treated lung tumors in terms of tumor location, CTV volume, and tumor density. An in-house developed toolkit within a treatment planning system allowed the shift of either the dose matrix or a shift of the beam isocenter with dose recalculation, to simulate setup errors and breathing motion. Setup shifts of different magnitudes (up to 10 mm) and directions as well as breathing with different peak-to-peak amplitudes (up to 10:5:5 mm) were modeled. The resulting dose–volume histograms (DVHs) were recorded and dose statistics were extracted.
Results: Generally, both the dose-shift and beam-shift methods resulted in calculated doses lower than the static planned dose, although the minimum (D98%) dose exceeded the prescribed dose in all cases, for setup shifts up to 5 mm. The dose-shift method also generally underestimated the dose compared with the beam-shift method. For clinically realistic systematic displacements of less than 5 mm, the results demonstrated that in the minimum dose region within the CTV, the dose-shift method was accurate to 2% (root-mean-square error). Breathing motion only marginally degraded the dose distributions.
Conclusions: Averaged over the patients and shift directions, the dose-shift approximation was determined to be accurate to approximately 2% (RMS) within the CTV, for clinically relevant geometrical uncertainties for SBRT of lung tumors.
Introduction
Inter- and intra-fractionation geometrical uncertainties, such as setup errors and breathing motion, can result in a delivered dose to the target different from the estimated dose from the static treatment plan. There have been numerous studies over the last two decades investigating various aspects of this phenomenon and its management. Schwarz et al. [Citation1] provide a useful summary in the context of SBRT of lung. There is, however, an apparent inconsistency between publications regarding calculations of dose distributions modified by geometrical uncertainties. In many modeling studies, a stationary dose-cloud approximation has been assumed, in which a geometrical shift is approximated by a shift of the static dose distribution [Citation2]. The integral dose to the target volume over many geometrical positions can then be considered a convolution of the static dose with the distribution of geometrical errors. The computational burden of such an approach is relatively low. While a valid assumption in many circumstances, Admiraal et al. [Citation3], Mexner et al. [Citation4], and Guckenberger et al. [Citation5] have shown using four-dimensional computed tomography (4DCT) that the high-dose region can track the moving tumor. This is a phenomenon arising from the differential in density between the tumor and its surrounding. To account for tissue inhomogeneities, a more accurate method considering geometrical uncertainties is fluence convolution, where the fluence is convolved with the distribution of geometrical errors before the dose calculation [Citation6]. In studies comparing dose-convolution with fluence-convolution, Beckham et al. [Citation6] demonstrated underestimation of the dose perturbation close to the inhomogeneity interfaces by the dose-convolution method, while Chetty et al. [Citation7] observed moderate dose differences within lung tumors.
Direct simulation is another method, where the beams are repositioned at each possible discretized geometric position and dose is recalculated. The total dose is a weighted summation of the recalculated dose at each position [Citation8]. In contrast to the results of the studies cited above, Craig et al. [Citation8] have shown in a phantom study that the differences between static dose-convolution and direct simulation are negligible despite the presence of inhomogeneities. Lax et al. [Citation9] have also shown that static dose-convolution of stereotactic body radiation therapy (SBRT) plans has acceptable agreement with dose recalculation at different breathing phases, when simulating breathing motions of lung tumors. Further, Engelsman et al. [Citation10] have demonstrated only small perturbations to the dose distribution from tumor shifts with film measurements in a lung phantom. The references cited deal with conventional radiotherapy with homogeneous dose distribution [Citation6–8,Citation10], and except for one dealing with SBRT with heterogeneous dose distribution [Citation9], not much has been published regarding heterogeneous dose distributions.
Given the complex picture emerging from the background literature, the degree of validity of the dose-shift method is unclear within lung SBRT. In this study, the dose-shift method (DSh) is compared with the beam–shift method with dose recalculation at each geometric position (BSh). The CTV dose is investigated for a set of ten SBRT lung patients, considering setup errors and breathing motion. These patients are representative of the variety treated in our clinic.
Material and methods
Simulation of geometrical uncertainties
Simulations of delivered dose were performed within the RayStation treatment planning system (RaySearch Laboratories AB, Stockholm, Sweden). A toolkit was developed in-house using IronPython and the numpy and scipy python libraries. The toolkit allowed the user to (i) shift a dose matrix (DSh method), or (ii) shift the beam (BSh method). In the former case, the delivered dose was estimated by convolving the planned dose matrix with the breathing trace and then displacing it according to a specified setup shift. In the latter case, for each breathing phase, the combined shift due to respiration and setup shift was applied as an isocenter shift and the dose distribution was recalculated. The delivered BSh dose was then the weighted sum of the doses in each phase. A Python script was written to automate comparisons of both possibilities.
Setup shifts
For simplicity, setup errors were simulated in one direction at the time, keeping the setup errors in the other two directions at zero. In each of the longitudinal, lateral and vertical directions, the following setup errors were modeled: −10, −5, −2, 0, 2, 5, and 10 mm. Positive shift values represented cranial, leftwards, and anterior shifts of the tumor (i.e. patient). Only translations were considered in this study, not rotations nor deformations. It was further assumed that the setup error was the same for all fractions of the treatment, i.e. a systematic error.
Note that in a review of 50 patients treated with SBRT for 65 lung tumors at our center over a total of 195 fractions (see Figure S1 in electronic supplement), the median setup error (as evaluated from cone-beam computed tomography (CBCT)) was 2 mm in the longitudinal direction (3% exceeded 10 mm), 2 mm in the lateral direction (1% exceeded 10 mm), and 2 mm in the vertical direction (0% exceeded 10 mm). Note that these data include mispositioning of the patients in the frame, errors in setup to correct stereotactic coordinates, tumor baseline shift and the phase of breathing captured during the three-dimensional treatment planning CT. The data thus include the setup errors prior to couch correction from CBCT imaging, and gives an indication of the typical range of geometrical errors.
Breathing motion
Arriving at a plausible breathing trace for simulations is fraught with difficulties. In many studies, a trace of the Lujan form [Citation11] has been assumed, with highest probability of positioning at the extreme phases of the breathing cycle. Yet imaging studies of breathing motion at our center and elsewhere [Citation12–14] have indicated that the dwell time at extreme positions is less than would be indicated by a single Lujan trace. What is also clear is that there will generally be correlations between displacements in the three dimensions. In absence of complete information on respiration in our patient group, a single representative trace was constructed as follows.
Projection images from a total of 16 CBCT scans were evaluated for nine patients treated with SBRT for liver tumors with implanted gold markers. The motion pattern of the implanted gold marker was tracked in the longitudinal direction with an in-house developed Matlab script (MathWorks Inc, Natick, MA, USA). A probability distribution function (PDF) was constructed for each patient. An average PDF was then calculated which was smoothed and reduced to 15 phase bins. Lateral and vertical motions could not be decoded from the two-dimensional CBCT projections. In the absence of information, a uniform PDF was assumed in these directions. See for the three PDFs. These data of breathing traces can be compared with data from Worm et al. [Citation14], also studying gold markers implanted in the liver. Although the breathing traces were extracted from liver rather than lung, they would be expected to have some correspondence. This is especially true of the caudal area of the lung, close to the diaphragm, where tumor motion amplitudes are typically largest (as assessed by 4DCT).
Figure 1. Smoothed average breathing PDF in longitudinal direction and uniform breathing PDF in lateral and vertical directions.
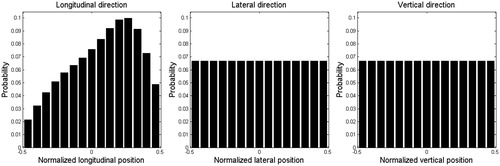
For this study, a breathing trace was constructed from the PDFs, using cumulative probability as a surrogate for time. First, hysteresis effects were ignored: it was assumed tumors moved along the same path at inspiration and expiration. This assumption was made for simplicity although it is known to be violated for some tumors [Citation13]. Second, it was assumed that the traces in each direction started synchronized at one end point, reached the other end point synchronously and returned to the first end point at the same time, in a single breathing cycle. Three sets of peak-to-peak amplitudes (longitudinal:lateral:vertical) were simulated. These were: 10:5:5 mm, 5:2.5:2.5 mm, and 0:0:0 mm (no motion), none of which exceed the CTV to planning target volume (PTV) margin. Note that in a review of 163 patients treated with SBRT for lung tumors at our center (data not published), the median breathing motion amplitude in the longitudinal direction was 5 mm (11% exceeded 10 mm) and in the transversal directions was 3 mm (7% exceeded 5 mm). Thus, for the majority of SBRT treated lung tumors at out clinic, the breathing motion amplitudes are within the limits simulated in this study.
Patient-specific amplitudes were not used in these simulations. Note that the aim of this study was not to estimate the dose to any specific patient, but instead to estimate the differences between the DSh and BSh methods with clinically relevant breathing amplitudes. Thus, to a large extent, the precise trace used was irrelevant. It was verified that that use of Lujan traces with the same amplitudes did not change the conclusions of the work.
Dose distributions and metrics
Dose–volume histograms (DVH) for the CTV were calculated for both the DSh and the BSh methods, with different breathing amplitudes and setup shifts. From data retrieved from the DVHs, dose discrepancies between DSh and BSh methods were evaluated in terms of D98%, D50%, and D2%. Mean value and root-mean-square (RMS) of dose differences for all patients were calculated. The latter metric is sensitive to both random and systematic differences between methods, while the former permits the systematic component to be distinguished. The DSh CTV volume receiving the BSh D98%, D50%, and D2% values were also determined. In particular, D98% was of interest as minimum dose to the CTV is expected to be a major factor for the probability of eradicating the clonogenic tumor cells, and thus to end up with local control.
Patients and planning
Included in this study were 10 tumors in 10 patients treated with SBRT for primary lung cancer or lung metastases. The study was approved by the regional ethical committee (2014/1813-31/1, 2014/11/12). Patients were selected to represent the variety of lung tumors treated with SBRT at our department, in terms of: location (cranial, middle, or caudal part of the lungs), CTV volume, tumor density (from solid tumors with distinct edges, to diffuse tumors with blurred edges or low density regions inside the tumor), and tumor shape (round or irregular). See Table S1 in the electronic supplement for further details. The CTV was generally defined as the gross tumor volume (GTV) including tumor spikes and diffuse growth at its borders, adding a maximum of a 1–2 mm margin to the GTV at lung window. All patients were fixated with a stereotactic body frame, and two of them were treated with abdominal compression. Around the CTV, margins of 10 mm in the longitudinal direction and 5 mm in the transversal directions were added to create the PTV. The treatment technique is described in detail by Lax et al. [Citation15], except for the addition of CBCT image-guidance in the setup process. Prescribed dose to the periphery of the PTV was 15 Gy ×3, corresponding to approximately the 67% isodose line. Treatment plans were created in Eclipse (Varian, Palo Alto, CA, USA) using 5–6 static fields with 6 MV photon beams calculated with the Analytical Anisotropic Algorithm (AAA) dose calculation algorithm. The plans were exported to RayStation (RaySearch Laboratories AB, Stockholm, Sweden) and were recalculated with the Collapsed Cone dose calculation algorithm with the same beam-data and with a voxel size of 1.5 × 1.5 × 1.5 mm3. It was verified that the static dose distributions recalculated in RayStation provided acceptable plans. Transversal cross-sections of the patient specific planned dose distributions are presented in the electronic supplement (Figures S2–S11).
Results
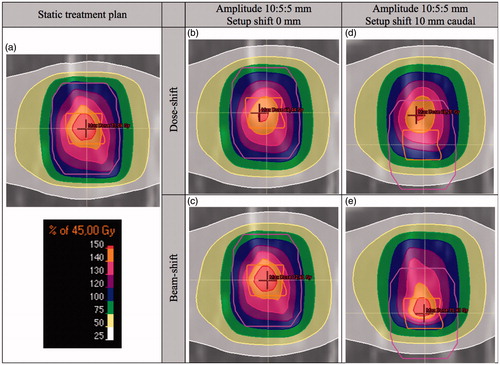
To graphically illustrate differences between the DSh and BSh methods, the dose distributions generated with the two methods are visualized in (for patient 4). The high-dose region in the static treatment plan () was blurred and reduced by the breathing motion when using the DSh method (). The high-dose region was more comparable with the static treatment plan in the BSh dose distribution (). When further adding a setup shift of 10 mm, the high-dose region was also partly shifted outside the CTV with the DSh approximation (). In contrast, the high-dose region followed the tumor density in the BSh dose distribution ().
Figure 2. Static dose distribution and dose distributions considering breathing motion and setup shifts, calculated with the DSh and BSh methods, for patient 4, in frontal projection. The figure is provided in color in the digital version. The CTV (inner structure) and PTV (outer structure) are delineated.
Subsequently, the results presented will primarily be of statistics derived from the whole patient cohort. For the purpose of comprehensiveness, however, an electronic supplement is provided with this paper, including detailed results for each simulated patient (Figures S12–S21).
Some observations supported by the supplementary data are that
In line with expectations, the DVHs generally demonstrate lower estimated delivered dose (with either DSh or BSh methods) compared to the static treatment plan. This was not always the case, however (see Figures S12a–S21a in the electronic supplement).
The calculated delivered doses show that the D98% exceeded the prescribed dose with setup shifts of 5 mm (see Figures S12b–S21b in the electronic supplement). For setup shifts of 10 mm, however, this was not assured.
The RMS and mean dose discrepancies between DSh and BSh methods for the 10 patients are shown in and , respectively. The statistics are presented for D98%, D50%, and D2%, for all simulated breathing amplitudes and setup displacements. A numerical summary of some of the results presented in the figures and electronic supplement is given in . Observe that larger RMS dose discrepancies between the DSh and BSh methods are seen for larger setup shifts (see ). The results demonstrate that DSh method was accurate to within 4%, in terms of D98% dose in the CTV, even for systematic displacements of 10 mm. For more clinically realistic setup shifts up to 5 mm, the DSh method was accurate to approximately 2% (RMS, ).
Figure 3. RMS dose discrepancies between the DSh and BSh methods for D98% (top), D50% (middle) and D2% (bottom), in relation to the BSh dose, averaged over all patients, for different breathing amplitudes (10:5:5, 5:2.5:2.5, and no breathing) and for longitudinal (left), lateral (middle), and vertical (right) setup shifts. The figure in color is provided in the digital version.
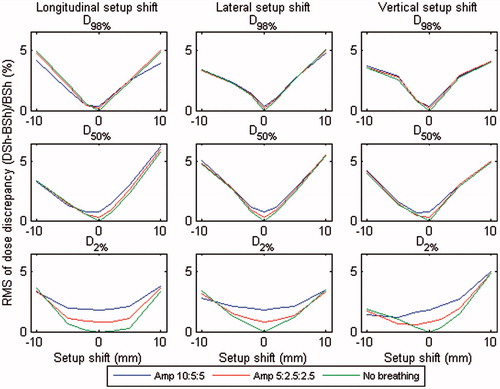
Figure 4. Mean dose discrepancies between the DSh and BSh methods calculated at D98% (top), D50% (middle) and D2% (bottom), in relation to the BSh dose ((DSh-BSh)/BSh), averaged over all patients, for different breathing amplitudes (10:5:5, 5:2.5:2.5, and no breathing) and for longitudinal (left), lateral (middle), and vertical (right) setup shifts. Error bars indicate the standard error on the mean dose discrepancy. The figure in color is provided in the digital version.
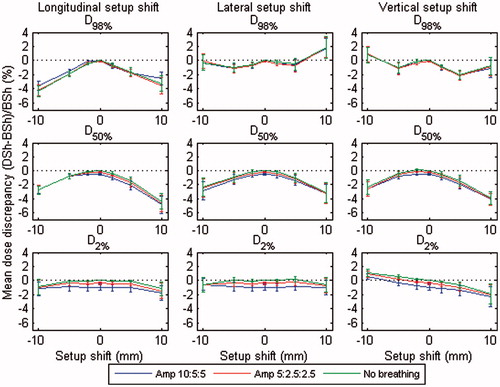
Table 1. For D98%, D50%, and D2%, values of maximum and mean dose discrepancies are given, as well as RMS dose discrepancy, in relation to the BSh dose. Also given are the maximum and mean volume differences. Data presented for setup shifts of 2, 5, and 10 mm (in any/all directions), for different simulated breathing motion amplitudes.
The DSh method generally underestimated the dose compared with the BSh method, especially at larger setup shifts. This is demonstrated in and indicates a systematic difference between the calculation methods. However, there were counter-examples to this trend. For example, the DSh method predicted a higher mean D98% dose than BSh at a 10 mm setup shift in lateral and vertical directions. For further detail, we refer the reader to the patient specific dose data (Figures S12c–S21c in the electronic supplement).
The volume discrepancies in show that the DSh method generally also underestimates the CTV volume covered by a specified dose level, compared with the BSh method. This is consistent with the DSh method generally underestimating the delivered dose. The volume discrepancy is an issue relevant for tumor control probability (TCP) calculations, but is not further investigated in this study.
The results also show that breathing motion was of minor importance compared with setup shifts. This is in agreement to the literature [Citation10,Citation16] on requirements on the PTV margins to deliver a specified dose to the CTV. The effects were most pronounced at small setup shifts, especially when looking at D2%.
Discussion
This study was to some extent prompted by the apparent discrepancy in the literature between claims for the validity of the dose-cloud assumption of applying dose shifts and the dose tracking effects observed by Admiraal et al. [Citation3] and Mexner et al. [Citation4] for lung tumor treatments. Our results have demonstrated that even in lung SBRT, the mean and RMS errors introduced with the DSh assumption are relatively small, when considering the dose to the CTV. The DSh approximation was determined to be accurate to approximately 2% (RMS), for clinically relevant breathing amplitudes and setup errors of up to 5 mm. Larger errors are unrealistic with daily image-guidance based on tumor position (and unlikely even without such guidance: see setup shifts statistics quoted in Material and Methods section). This means the DSh (or “dose-cloud”) assumption is quite adequate for many purposes.
Breathing motion was demonstrated to have only minor effects (where breathing was considered to be consistent at planning and delivery and no intra-fraction base-line shifts occurred). We note that patients had CTV–PTV margins of 10 mm in longitudinal direction and 5 mm in transversal directions. Breathing motion amplitudes were only simulated up to 10:5:5 mm (larger amplitudes would have led to increased CTV–PTV margins at our clinic).
A limitation of this study was that deformations of the body were not considered. While the rigid shifts of the isocenter in the BSh method accurately reflect true setup errors, deformations due to tumor baseline shift and breathing motions are not accounted for. It is difficult to assess the likely impact of deformations on the dose, as the issue depends on the patient and the plan in a complex way and requires additional information. In fact, it has been suggested that assuming a rigid shift to account for breathing generally is accurate to within 2%, even with very large breathing motions [Citation17,Citation18]. What is clear, however, is that the BSh method accounts for the tissue density changes between tumor and surroundings, which the DSh method does not. It is this effect which is seen to dominate in the observations of Admiraal et al. [Citation3] and Mexner et al. [Citation4]. For this reason, the results presented here are considered informative on the likely accuracy of the DSh method.
We note that it would have been of interest to perform dose calculations on different 4DCT phases, as in the studies by Admiraal et al. [Citation3], Mexner et al. [Citation4], and Guckenberger et al. [Citation5]. In principle, this would have yielded a superior ground-truth to the BSh method. However, there are some practical challenges with this approach. First, it would have required deformation of the dose from the different phases to a common reference frame. Second, there is a finite number of phases and 4DCT has been observed to underestimate breathing amplitudes [Citation19]. Third, the phase-binning of 4DCT data can result in image artifacts, especially for patients with large breathing amplitudes. Therefore, in fact, it is unclear as to the accuracy with which 4DCT-based dose calculation does represent the ground-truth. Further, when using 4DCT for dose calculations, it is not possible to apply an arbitrary breathing trace and tumor baseline shift to estimate delivered dose: the deformations are assumed to be those reconstructed from the earlier 4DCT scan. For these reasons, we adopted the simplicity and flexibility of the BSh method in this study, despite its limitations.
A further limitation of our methodology was that of voxel size. It was found that for small tumors, even with the smallest voxel size that could be selected due to memory limitations of running the Python script, the voxel dimension was not negligible compared to the size of the tumor. This lead in some instances to sudden transitions in DVH curves. Also, quite generally, the flatness of the DVHs in the low-dose regions made the precise values of D98% sensitive to the discretization of the voxel matrix. As these factors only exaggerate the differences between the DSh and BSh methods, we do not consider their effects to invalidate our conclusions.
Guckenberger et al. [Citation5] suggested that dose accumulation based on shifts of a static dose cloud (i.e. DSh) is an inappropriate tool for accurate estimation of delivered dose in SBRT. We note, however, that their study included relatively large breathing amplitudes (a mean of 14 mm) and considered doses at extreme displacements (between end-exhale and inhale). When averaged over the whole breathing cycle, any error due to a dose-shift assumption should be substantially less than that between end-exhale and inhale. This last conclusion is supported by the relatively small magnitudes of disagreement between DSh and BSh methods displayed in and (<4%).
Beckham et al. [Citation6] compared dose-convolution and fluence-convolution considering random setup errors in a phantom. They determined that in homogeneous media the two methods are similar, but close to lung/water or bone/water interfaces the dose-convolution method underestimates the dose perturbation. This is consistent with our finding that the DSh method generally predicted slightly lower doses for the group of lung SBRT patients (see ). Chetty et al. [Citation7] compared dose-convolution and fluence-convolution (cf. DSh and BSh) accounting for target breathing motion with patient-specific amplitudes for three patients treated with conventional RT in the lungs. The authors found up to a 7% dose discrepancy (except at the surface of the patients), but the areas of largest discrepancy were predominately outside the CTV. They declared that fluence-convolution tends to estimate higher doses than dose-convolution, an observation that is again consistent with our results. Interestingly, Chetty et al. found higher doses with the two convolution methods compared with the static treatment plan for one of their patients. They explained this to be an effect of the hot-spots dominating the overall dose in the PTV. This illustrates the point that the unique peculiarities of a specific plan can lead to results contradicting any “rules of thumb” derived from general trends. Our results generally determined lower doses with both the DSh and BSh methods compared to the static treatment plan (Figures S12a–S21a in electronic supplement), but there were exceptions.
Lax et al. [Citation9] simulated linear cranio-caudal breathing motion, comparing static dose-convolution with summation of doses recalculated at different breathing phases. They observed overestimation of the dose by the dose-convolution method at the tumor edge and underestimation of the central high dose. The results in this current study, however, suggest that the dose-shift method quite generally underestimates the dose in the CTV compared to the beam-shift method. Further, Panettieri et al. [Citation20] estimated uncertainties in calculated dose through direct simulation of breathing motion (up to ±16 mm) and systematic setup errors (2 and 4 mm) compared with the static treatment plan. They showed that at the periphery of the tumor the static treatment plan overestimates the dose up to approximately 10%, while the dose in the central parts is accurately estimated. Consistent with this, the current study demonstrated that the static treatment plan generally overestimates the delivered dose at the peripheral parts of the tumor and is more accurate in the central parts (Figures S12a–S21a in electronic supplement).
Note that the studies by Lax et al. [Citation9] and Panettieri et al. [Citation20] were performed using a phantom assuming the GTV as homogeneous water density with distinct edges surrounded by homogeneous lung equivalent material. The resulting errors are, therefore, likely to be somewhat larger compared with the clinical case, where there often is a more diffuse border of the tumor included in the expansion from the GTV to the CTV and where the tumor quite often also is surrounded by higher-density structures rather than only lung tissue. Pertinent to this point is the study by Craig et al. [Citation8]. Those authors considered geometrical Gaussian uncertainties comparing dose-convolution and direct simulation, and reported a few percent dose error near interfaces for phantoms with inhomogeneities. However, excellent agreement was shown between the two methods for the CTV DVH in a clinical lung case. They concluded that dose errors with the convolution method when inhomogeneities are present appear to be negligible.
A strength of this current study is the use of a sizable set of patients representative of the patient variation in clinical practice. This has provided insight into the magnitude of errors in the DSh method in clinically relevant scenarios and assisted in the interpretation of the sometimes contradictory observations in the literature. Following on and encouraged by this study, the DSh method has been applied in our center in a forthcoming publication to simulate SBRT lung deliveries for a population of patients. Its computational ease compared with the BSh method lends itself to such studies. Note that a more sophisticated alternative has been developed by Tilly et al. [Citation21]. The authors have developed a fast dose algorithm to account for dosimetric effects of geometrical uncertainties through applying setup uncertainties and intra-fractional motion to the pre-calculated primary and scatter dose components. Compared with a full dose calculation, this method has a high agreement (within 2%/2 mm) and is approximately 1000 times faster. However, this method is not yet commercially available.
Conclusions
The dose-shift method was compared with the beam-shift method (the latter with dose recalculation in a treatment planning system), to account for geometrical uncertainties for SBRT in the lungs. For high accuracy in estimation of patient-specific doses considering geometrical uncertainties, it is advisable to use the BSh method. When averaged over a patient population, however, the mean and RMS errors introduced with the DSh assumption are relatively small. Averaged over the patients and shift directions, the dose-shift approximation was determined to be accurate to approximately 2% (RMS) within the CTV, for clinically relevant breathing amplitudes and setup errors up to 5 mm.
IONC_1310395_Supplement_Information.docx
Download MS Word (2.7 MB)Acknowledgments
Gratefully acknowledged is Erik Wåhlin for valuable contribution of data of breathing motion patterns.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Schwarz M, Cattaneo GM, Marrazzo L. Geometrical and dosimetrical uncertainties in hypofractionated radiotherapy of the lung: a review. Phys Med. 2017 [Feb 23]. DOI:10.1016/j.ejmp.2017.02.011
- Bortfeld T, Jiang SB, Rietzel E. Effects of motion on the total dose distribution. Semin Radiat Oncol. 2004;14:41–51.
- Admiraal MA, Schuring D, Hurkmans CW. Dose calculations accounting for breathing motion in stereotactic lung radiotherapy based on 4D-CT and the internal target volume. Radiother Oncol. 2008;86:55–60.
- Mexner V, Wolthaus JW, van Herk M, et al. Effects of respiration-induced density variations on dose distributions in radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys. 2009;74:1266–1275.
- Guckenberger M, Wilbert J, Krieger T, et al. Four-dimensional treatment planning for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:276–285.
- Beckham WA, Keall PJ, Siebers JV. A fluence-convolution method to calculate radiation therapy dose distributions that incorporate random set-up error. Phys Med Biol. 2002;47:3465–3473.
- Chetty IJ, Rosu M, McShan DL, et al. Accounting for center-of-mass target motion using convolution methods in Monte Carlo-based dose calculations of the lung. Med Phys. 2004;31:925–932.
- Craig T, Battista J, Van Dyk J. Limitations of a convolution method for modeling geometric uncertainties in radiation therapy. I. The effect of shift invariance. Med Phys. 2003;30:2001–2011.
- Lax I, Panettieri V, Wennberg B, et al. Dose distributions in SBRT of lung tumors: comparison between two different treatment planning algorithms and Monte-Carlo simulation including breathing motions. Acta Oncol. 2006;45:978–988.
- Engelsman M, Damen EM, De Jaeger K, et al. The effect of breathing and set-up errors on the cumulative dose to a lung tumor. Radiother Oncol. 2001;60:95–105.
- Lujan AE, Larsen EW, Balter JM, et al. A method for incorporating organ motion due to breathing into 3D dose calculations. Med Phys. 1999;26:715–720.
- George R, Vedam SS, Chung TD, et al. The application of the sinusoidal model to lung cancer patient respiratory motion. Med Phys. 2005;32:2850–2861.
- Seppenwoolde Y, Shirato H, Kitamura K, et al. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53:822–834.
- Worm ES, Hoyer M, Fledelius W, et al. Three-dimensional, time-resolved, intrafraction motion monitoring throughout stereotactic liver radiation therapy on a conventional linear accelerator. Int J Radiat Oncol Biol Phys. 2013;86:190–197.
- Lax I, Blomgren H, Larsson D, et al. Extracranial stereotactic radiosurgery of localized targets. J Radiosurg. 1998;1:14.
- van Herk M, Witte M, van der Geer J, et al. Biologic and physical fractionation effects of random geometric errors. Int J Radiat Oncol Biol Phys. 2003;57:1460–1471.
- Mexner V, Wolthaus J, Nijkamp J, et al. Effects of respiration-induced anatomy variations on dose distributions [Abstract]. Radiother Oncol. 2007;84(Suppl. 1):S75.
- Sonke JJ, Rossi M, Wolthaus J, et al. Frameless stereotactic body radiotherapy for lung cancer using four-dimensional cone beam CT guidance. Int J Radiat Oncol Biol Phys. 2009;74:567–574.
- Nielsen TB, Hansen CR, Westberg J, et al. Impact of 4D image quality on the accuracy of target definition. Australas Phys Eng Sci Med. 2016;39:103–112.
- Panettieri V, Wennberg B, Gagliardi G, et al. SBRT of lung tumours: Monte Carlo simulation with PENELOPE of dose distributions including respiratory motion and comparison with different treatment planning systems. Phys Med Biol. 2007;52:4265–4281.
- Tilly D, Ahnesjö A. Fast dose algorithm for generation of dose coverage probability for robustness analysis of fractionated radiotherapy. Phys Med Biol. 2015;60:5439–5454.
