Abstract
Background: The European Organization for Research and Treatment of Cancer (EORTC) 62012 study was a Phase III trial of doxorubicin versus doxorubicin–ifosfamide chemotherapy in 455 patients with advanced soft tissue sarcoma (STS). Analysis of the main study showed that combination chemotherapy improved tumor response and progression-free survival, but differences in overall survival (OS) were not statistically significant. We analyzed factors prognostic for tumor response and OS, and assessed histological subgroup and tumor grade as predictive factors to identify patients more likely to benefit from combination chemotherapy.
Methods: Central pathology review was performed by six reference pathologists. Gender, age, performance status, time from first presentation with sarcoma to starting palliative chemotherapy, tumor grade, histological subgroup, primary tumor site involvement, and sites of metastases were assessed as prognostic factors.
Results: Three hundred and ten patients were included in this study. Discordance between local and central pathology opinion of tumor histology and tumor grade was observed in 98 (32%) and 122 (39%) cases, respectively. In multivariate analysis, liposarcoma patients had improved tumor response compared to other histological subgroups, whilst patients with metastases other than lung, liver or bone had a poorer response [odds ratio (OR) 0.42, 95% confidence interval (CI) 0.23–0.78; p = 0.006]. Patients with bone metastases had reduced OS [hazard ratio (HR) 1.56, 95% CI 1.16–2.09; p = 0.003]. By central pathology review, patients with undifferentiated pleomorphic sarcoma (UPS) had improved tumor response and OS with doxorubicin–ifosfamide compared to single-agent doxorubicin (OR 9.90, 95% CI 1.93–50.7 and HR 0.44, 95% CI 0.26–0.79, respectively). Grade III tumors had improved response with combination chemotherapy but there was no interaction between chemotherapy and grade on OS.
Conclusions: Prospective central pathology review of tumor histology should be integrated into future STS clinical trials. Doxorubicin–ifosfamide may be most appropriate for young, fit patients with poorly differentiated Grade III tumors including UPS.
Introduction
Soft tissue sarcomas (STS) are a group of rare aggressive tumors of mesenchymal origin, separated into over 50 different subtypes by histological and molecular classifications [Citation1,Citation2]. Chemotherapy is the mainstay of treatment for patients with unresectable metastatic disease, and is usually administered with palliative intent. Doxorubicin and ifosfamide have single-agent activity in STS [Citation3,Citation4], but the role of doxorubicin–ifosfamide combination has been less certain. The European Organization for Research and Treatment of Cancer (EORTC) 62012 study was a multi-centre randomized Phase III trial of first-line single-agent doxorubicin versus intensified doxorubicin–ifosfamide chemotherapy for young, fit patients with advanced intermediate or high-grade STS [Citation5]. Combination chemotherapy was associated with a significantly higher tumor response rate (complete + partial response, 26 versus 14%; p < .0006) and improved progression-free survival [PFS, hazard ratio (HR) 0.74, 95% confidence interval (CI) 0.60–0.90; p = .003], but overall survival (OS) was not significantly different (HR 0.83, 95% CI 0.67–1.03; p = .076). Furthermore, combination chemotherapy was associated with significantly more toxicity (Grade 3–4 febrile neutropenia 46 versus 13%; p < .0001). The study authors concluded that single-agent doxorubicin was appropriate for the majority of patients with advanced STS; however, combination chemotherapy was justified for select patients in whom the primary aim of treatment was tumor shrinkage, to alleviate symptoms or to enable local disease control by subsequent surgery or radiotherapy.
A previous meta-analysis of seven heterogeneous EORTC-led clinical trials of first-line anthracycline-based chemotherapy for advanced STS-reported younger age, good performance status (PS) and absence of liver metastases as prognostic of both improved tumor response to chemotherapy and OS [Citation6]. Higher tumor grade and liposarcoma histology were other factors associated with improved tumor response to chemotherapy, whilst low tumor grade and longer time elapsed from initial diagnosis of sarcoma to starting first-line chemotherapy were associated with improved OS.
We performed an analysis of the EORTC 62012 study to validate factors prognostic of tumor response to chemotherapy and OS in patients with advanced STS treated in a contemporary prospective randomized Phase III clinical trial. We then explored histological subtype and tumor grade as predictive factors to identify patient subgroups more likely to benefit from treatment with combination chemotherapy.
Methods
Patients included in the subgroup analysis
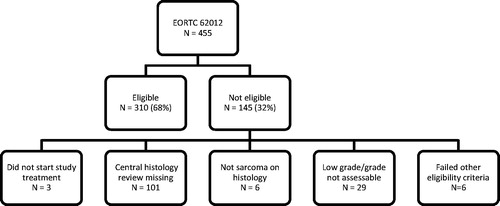
Four hundred and fifty-five patients were recruited to the EORTC 62012 study (NCT00061984). The detailed eligibility criteria for the EORTC 62012 study have previously been published [Citation5], including age ≤60 years, WHO PS 0 or 1, and intermediate or high-grade STS by local pathology opinion. Patients who received at least one cycle of chemotherapy were eligible for the subgroup analysis. A central pathology review of tumor histology and tumor grade was mandated in the trial protocol and performed by six expert STS pathologists according to the World Health Organization 2013 classification of tumors of soft tissue and bone [Citation1] and the French Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) system [Citation7], respectively. Cases without central pathology review, or without sarcoma histology, or where tumor grade was low or not assessable by central pathology review, or who did not meet other eligibility criteria for the main study were excluded (). The study population thereby consisted of 310 patients with characteristics broadly similar to the main study population ().
Table 1. Patient characteristics.
Histological subtypes were pooled for analysis into liposarcoma, leiomyosarcoma, synovial sarcoma, undifferentiated pleomorphic sarcoma (UPS) or ‘other’ subgroups. Gender, age, PS, time elapsed from initial presentation with sarcoma to starting palliative chemotherapy, tumor grade, histological subgroup, primary tumor site involvement and site of metastases (liver, lung, bone and ‘other’) were assessed as factors prognostic for tumor response to chemotherapy and OS. Patients were included in the prognostic factor analysis based on central pathology review.
Histological subgroup and tumor grade were then assessed as factors predictive of improved tumor response and OS with combination chemotherapy. In this exploratory analysis, histological subgroup and tumor grade were analyzed according to both local and central pathology assignment.
Statistics
Response to chemotherapy was reported based on local investigator assessment according to RECIST 1.0 [Citation8]. OS was computed from the date of randomization in the study to the date of death. Patients still alive at the time of the analysis were censored at their last follow-up date or the clinical trial cut-off date, whichever occurred first. Analyses for response rate (complete + partial response) were performed using logistic regression; analyzes for OS were performed using Cox regression models. Factors included in the final multivariate models were identified using stepwise selection. A significance level of 0.15 was required to include a factor in the multivariate model, and a significance level of 0.05 was required for a factor to stay in the model.
Results
Central pathology review of tumor histology was available for 354/455 cases (78%). Discordance with local assessment was observed in 118 cases (33%), including six patients who did not have STS histology on central review. Central pathology review of tumor grade was available for 339/455 cases (75%). Discordance with local assessment was observed in 141 cases (42%). After excluding patients that failed other eligibility criteria, 310 patients were included in the subgroup analysis. Of these 310 patients, discordance between local and central pathology assessment of tumor histology and tumor grade was observed in 98 (32%) and 122 (39%) cases, respectively. Consistent with the main study results, combination chemotherapy was associated with improved tumor response [odds ratio (OR) 2.44, 95% CI 1.38–4.31; p = .002], but OS was not significantly different (HR 0.82, 0.64–1.04; p = .105).
Prognostic factor analysis
In multivariate analysis, gender, age, PS, time from first presentation with sarcoma to starting palliative chemotherapy, tumor grade, histological subgroup, primary tumor site involvement, and sites of metastases were assessed as potential factors prognostic for tumor response to chemotherapy and OS. Central pathology review of histology and tumor grade were used for this analysis.
In multivariate analysis, liposarcoma histology and ‘other’ metastatic disease sites were prognostic for tumor response to chemotherapy (). Patients with liposarcoma had improved tumor response to chemotherapy compared to other histological subgroups (overall p = .014). The lungs are the most common site of metastases for patients with STS, and in this analysis were present in 227/310 (73.2%) cases (). Sites of metastatic disease other than lung, liver and bone were involved in 185/310 (59.7%) cases, and included lymph node metastases (92 cases), skin metastases (9 cases) and other soft tissue metastases (77 cases). Patients with metastases present at ‘other’ sites had poorer tumor response to chemotherapy compared to patients that did not (OR 0.42, 95% CI 0.23–0.78; p = .006). Grade III tumors were associated with improved tumor response to chemotherapy, but this was not statistically significant (OR 1.41, 95% CI 0.75–2.65).
Table 2. Prognostic factors for best overall response (CR + PR): multivariate analysis stratified by treatment.
In multivariate analysis, PS 1 (HR 1.39, 95% CI 1.07–1.80; p = .013), shorter time from initial presentation with sarcoma to starting palliative chemotherapy (HR 1.43, 95% CI 1.02–2.00; p = .020), and presence of bone metastases (HR 1.45, 95% CI 1.01–2.09; p = .046) were associated with reduced OS. However, only bone metastases remained statistically significant (HR 1.56, 95% CI 1.16–2.09; p = .003) in the final reduced model ().
Table 3. Prognostic factor analysis for OS: multivariate analysis stratified by treatment.
Predictive factor analysis
Tumour grade (Grade II or III) and histological subtype, grouped into liposarcoma, leiomyosarcoma, synovial sarcoma, UPS, or ‘other’, were assessed as predictive factors. Outcomes differed depending on local or central pathology assignment of histological subtype (). By local pathology assessment of histology, synovial sarcomas and ‘other’ subgroups had a higher response rate with combination chemotherapy compared to single-agent doxorubicin [43.5 versus 11.1% (OR 6.15, 95% CI 1.43–26.39) and 29.0 versus 10.5% (OR 3.48, 95% CI 1.27–9.53) for synovial sarcoma and ‘other’, respectively], whilst tumor response rates for liposarcoma, leiomyosarcoma and UPS subgroups did not differ significantly by treatment arm. In contrast, by central pathology assessment, the UPS subgroup had a higher response rate with combination chemotherapy than with single-agent doxorubicin [42.3 versus 6.9% (OR 9.90, 95% CI 1.93–50.7)], but response did not differ significantly between treatment arms for liposarcoma, leiomyosarcoma, synovial sarcoma or ‘other’ subgroups. Analysis of OS by local pathology assessment showed no interaction between histological subgroup and treatment arm, whilst patients with UPS by central pathology review had improved OS with combination chemotherapy compared with single-agent doxorubicin (HR 0.44, 95% CI 0.26–0.79) ().
Figure 2. Interaction of histological subtype with treatment on OS (A: central pathology review; B: local pathology review).
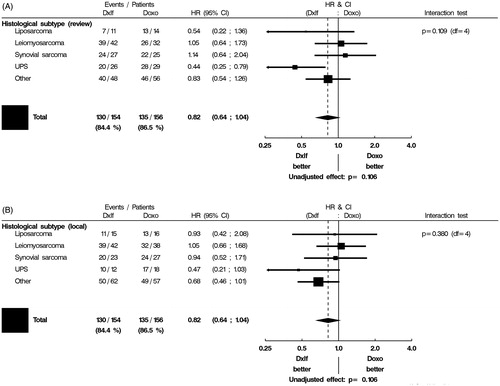
Table 4. Interaction of histological subtype on response to treatment (A: local pathology assessment; B: central pathology assessment).
Irrespective of local or central pathology assessment, Grade III tumors had an improved response rate with combination chemotherapy compared with single-agent doxorubicin (OR 2.93, 95% CI 1.30–6.61 and 3.64, 95% CI 1.72–7.70 by local and central pathology assessment, respectively). Response rate in Grade II tumors by either local or central pathology assessment did not differ significantly by treatment arm. No interaction between treatment arm and tumor grade was identified in OS analysis, irrespective of local or central pathology assessment of grade.
Discussion
The EORTC 62012 was a multi-centre randomized Phase III trial of first-line single-agent doxorubicin versus intensified doxorubicin–ifosfamide chemotherapy for young, fit patients with advanced intermediate or high-grade STS [Citation5]. Patients were enrolled and the results of study reported based on local pathology assessment. We observed a substantial discordance between local pathology assessment and central pathology expert review of histological subtype and tumor grade. This degree of discordance is consistent with levels reported by other STS studies [Citation9–13]. STS pathology is highly complex, and the classifications of STS subtypes are constantly evolving. Despite the growing role of molecular pathology to facilitate diagnosis, the identification of STS subtypes still largely relies on interpretation of tumor morphology and immunohistochemistry. Central pathology review fulfills an important role in verifying the local pathology diagnosis. However, central pathology assessment is wholly dependent on the specimen submitted for review. In contrast, local pathology opinion may be refined by access to additional tumor samples and to clinical and radiological correlates. STS tumors contain areas of heterogeneity, and this may explain some of the discordance we observed between local and central pathology opinions.
Previously, clinical trials in STS included patients with a variety of different histological subtypes. As treatments of individual subtypes are progressively refined, clinical trials increasingly recruit patients with specific STS histological subtypes. The EORTC 62043 study, a single-arm Phase II trial of pazopanib in patients with advanced STS, e.g., assessed treatment response in four histological cohorts of STS (leiomyosarcoma, liposarcoma, synovial sarcoma and ‘others’) [Citation14]. On the basis of this study, patients with liposarcoma were excluded from the subsequent licensing Phase III PALETTE trial [Citation15]. Different conclusions could be drawn from our subgroup analysis of histological subtype as a predictive factor of response to combination chemotherapy in the EORTC 62012 study, dependent on whether local pathology or central pathology assessment of tumor histology was used. This analysis was exploratory, and was limited by small numbers of patients in each histological subgroup, but it highlights the importance of accurate pathology classification in STS studies to the interpretation of trial results. Local pathology assessment and central pathology review perform complementary functions, and our analysis suggests a role for incorporating mandatory prospective central pathology review into future trial protocols. This should become possible in practice as shared digital platforms become increasingly common.
Our analysis suggested that UPS, synovial sarcoma and ‘other’ histological subtypes were most likely to respond to treatment with combination chemotherapy. The histological subgroup labeled ‘other’ encompassed a pooled collection of rarer STS subtypes with diverse pathologies. Together, this subgroup represented a third (104/310) of all patients included in this analysis, which individually were too infrequent to be analyzed separately. Only UPS by central review classification had improved OS with combination chemotherapy. Interestingly, a contemporary study of peri-operative epirubicin + ifosfamide chemotherapy in localized high-risk STS of the trunk and extremities also reported improved OS outcomes in UPS compared to other histological subtypes [Citation16]. The lack of OS advantage with combination chemotherapy in synovial sarcoma and ‘other’ subtypes despite improved tumor response rates is consistent with a separate analysis of the EORTC 62012 study, which demonstrated that the absence of tumor progression and not the extent of disease remission defines prognosis in STS [Citation17]. Synovial sarcomas are considered to be chemosensitive tumors. Previous studies have suggested that synovial sarcomas have higher responses rates to chemotherapy than other STS subtypes, including improved response rates to regimens containing ifosfamide [Citation18]. UPS are aggressive high-grade tumors with no discernable histological differentiation [Citation19]. They are diagnosed by exclusion of other pleomorphic subtypes, including leiomyosarcoma and liposarcoma. Samples identified as UPS on central pathology review therefore include poorly differentiated STS subtypes, which have been re-classified on the basis of the submitted specimen. Such poorly differentiated tumors may have aggressive tumor biology that benefit more from combination chemotherapy. This would support the parallel observation that high-grade tumors were more likely to respond to combination chemotherapy than intermediate-grade lesions, although tumor grade did not influence OS.
We used central pathology assessment of tumor histology and tumor grade for the prognostic factor analysis, as this had been undertaken by a small panel of expert sarcoma pathologists. The prognostic factor analysis identified that liposarcoma histology was associated with improved tumor response rate compared to other histological subgroups. Previous studies have also suggested that liposarcomas are associated with a higher response rate [Citation6]. The liposarcoma subgroup consisted of disparate subtypes including dedifferentiated liposarcoma, pleomorphic liposarcoma and myxoid liposarcoma. Myxoid liposarcomas are considered chemosensitive, whilst dedifferentiated liposarcomas are considered less sensitive to chemotherapy. Unfortunately, the specific liposarcoma subtype present was not recorded centrally, and analysis to refine tumor response rate by liposarcoma subtype was not possible, although the small number of liposarcoma patients included in the study (25 cases by central pathology review) would have limited more detailed analysis.
We identified that the presence of metastases at sites other than lung, liver and bone was associated with poorer response to chemotherapy. In our study population, ‘other’ sites of metastatic disease were involved in almost 60% of cases. Previous large database studies of response to first-line palliative chemotherapy in advanced STS have reported the presence of metastatic disease at sites other than lung, liver and bone in ∼35% of cases [Citation20,Citation21], and have identified the presence of disease at ‘other’ metastatic sites as prognostic for adverse OS [Citation20].
PS is a well-established prognostic factor [Citation20]. The EORTC 62012 study recruited patients aged ≤60 years with WHO PS 0 or 1. It is therefore striking that PS was prognostic of OS despite eligibility criteria restricting the study population to young fit patients. Time between initial diagnosis of sarcoma and commencing palliative chemotherapy has previously been identified as prognostic [Citation6]. Patients with a shorter time to starting palliative chemotherapy from initial diagnosis (3–12 months) had worse OS. This cohort consisted of patients with poor tumor biology and rapidly progressive disease. A longer interval between initial diagnosis and starting chemotherapy (>12 months) implied less aggressive disease and was associated with improved OS, whilst patients presenting with metastatic disease (interval from initial diagnosis <3 months) represented a mix of these two patient populations. The presence of bone metastases was the only factor prognostic for OS in the final multivariate model. Bone metastases were reported in 44/310 (14.1%) patients included in the subgroup analysis. A previous multi-centre retrospective analysis identified bone metastases as a poor prognostic feature, and suggested routine use of bisphosphonate therapy for patients with metastatic bone disease to delay the onset of skeletal related events (e.g., pathological fracture, spinal cord compression or hypercalcaemia) [Citation22].
In summary, we performed an analysis of the EORTC 62012 study, a large Phase III trial of single-agent doxorubicin versus a doxorubicin–ifosfamide combination for advanced STS. This subgroup analysis highlights the importance of the sarcoma pathologist to the assessment of clinical trial outcomes. Single-agent doxorubicin remains standard of care first-line chemotherapy for patients with advanced STS. However, combination doxorubicin–ifosfamide is indicated for selected patients, and this analysis suggests combination treatment may be most appropriate to consider in patients ≤60 years old, PS 0 or 1, with poorly differentiated Grade III tumors including UPS.
Acknowledgments
The authors would like to thank all the patients and physicians who participated in the EORTC 62012 trial.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC; 2013.
- Hogendoorn PC, Collin F, Daugaard S, et al. Changing concepts in the pathological basis of soft tissue and bone sarcoma treatment. Eur J Cancer. 2004;40:1644–1654.
- Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. JCO. 1995;13:1537–1545.
- van Oosterom AT, Mouridsen HT, Nielsen OS, et al. Results of randomised studies of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with two different ifosfamide regimens in first- and second-line chemotherapy in advanced soft tissue sarcoma patients. Eur J Cancer. 2002;38:2397–2406.
- Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415–423.
- Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens - a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–157.
- Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. JCO. 1997;15:350–362.
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216.
- Thway K, Wang J, Mubako T, et al. Histopathological diagnostic discrepancies in soft tissue tumours referred to a specialist centre: reassessment in the era of ancillary molecular diagnosis. Sarcoma. 2014;2014:686902.
- Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13:1045–1054.
- Harris M, Hartley AL, Blair V, et al. Sarcomas in northwest England: I. histopathological peer review. Br J Cancer. 1991;64:315–320.
- Alvegård TA, Berg NO. Histopathology peer review of high-grade soft tissue sarcoma: the Scandinavian Sarcoma Group experience. JCO. 1989;7:1845–1851.
- Presant CA, Russell WO, Alexander RW, et al. Soft-tissue and bone sarcoma histopathology peer review: the frequency of disagreement in diagnosis and the need for second pathology opinions. The Southeastern Cancer Study Group experience. JCO. 1986;4:1658–1661.
- Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. 2009;27:3126–3132.
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886.
- Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. JCO. 2012;30:850–856.
- Grünwald V, Litière S, Young R, et al. Absence of progression, not extent of tumour shrinkage, defines prognosis in soft-tissue sarcoma - an analysis of the EORTC 62012 study of the EORTC STBSG. Eur J Cancer. 2016;64:44–51.
- Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer. 2010;46:72–83.
- Goldblum JR. An approach to pleomorphic sarcomas: can we subclassify, and does it matter? Mod Pathol. 2014;27(Suppl. 1):S39–S46.
- Penel N, Glabbeke MV, Mathoulin-Pelissier S, et al. Performance status is the most powerful risk factor for early death among patients with advanced soft tissue sarcoma: the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (STBSG) and French Sarcoma Group (FSG) study. Br J Cancer. 2011;104:1544–1550.
- Karavasilis V, Seddon BM, Ashley S, et al. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma. Retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112:1585–1591.
- Vincenzi B, Frezza AM, Schiavon G, et al. Bone metastases in soft tissue sarcoma: a survey of natural history, prognostic value and treatment options. Clin Sarcoma Res. 2013;3:6.