Abstract
Background: Adjuvant endocrine therapy has significantly improved survival of estrogen receptor α (ER)-positive breast cancer patients, but around 20% relapse within 10 years. High expression of ER-stimulated proteins like progesterone receptor (PR), Bcl-2 and insulin-like growth factor receptor I (IGF-IR) is a marker for estrogen-driven cell growth. Therefore, patients with high tumor levels of these proteins may have particularly good prognosis following adjuvant endocrine therapy.
Patients and methods: Archival tumor tissue was available from 1323 of 1396 Danish breast cancer patients enrolled in BIG 1-98, a randomized phase-III clinical trial comparing adjuvant letrozole, tamoxifen or a sequence of the two drugs. Immunohistochemical staining for ER, HER-2, PR, Bcl-2 and IGF-IR was performed and determined by Allred scoring (ER, PR and Bcl-2) or HercepTest (HER-2 and IGF-IR).
Results: Data on all five markers were available from 969 patients with ER-positive, HER-2-negative tumors. These patients were classified in ER activity groups based on the level of PR, Bcl-2 and IGF-IR. High ER activity profile was found in 102 patients (10.5%) and compared with the remaining patients, univariate and multivariate analysis revealed HR (95% CI) and p values for disease-free survival (DFS) of 2.00 (1.20–3.22), 0.008 and 1.70 (1.01–2.84), 0.04 and for the overall survival (OS) of 2.33 (1.19–4.57), 0.01 and 1.90 (0.97–3.79), 0.06, respectively. The high ER activity profile did not disclose difference in DFS or OS according to treatment with tamoxifen or letrozole (p = .06 and .09, respectively).
Conclusions: Stratifying endocrine-treated patients in ER activity profile groups disclosed that patient with high ER activity profile (10.5%) had significantly longer DFS and OS, and the profile was an independent marker for DFS. High ER activity is a marker for estrogen-driven tumor growth. We suggest further analyses to disclose whether the ER activity profile or other markers associated with estrogen-driven growth may be used to identify ER-positive high-risk breast cancer patients who can be spared adjuvant chemotherapy.
Background
Estrogen and the estrogen receptor α (ER) play a central role in development and growth of the majority of breast cancers. Breast tumors with ER-driven tumor growth are responsive to endocrine therapy and the antiestrogen tamoxifen, which competes with estrogen for binding to ER, has been the backbone of adjuvant endocrine therapy for early breast cancer [Citation1]. In postmenopausal patients, switching from tamoxifen to aromatase inhibitors, which block the conversion of testosterone to estradiol, has further reduced the risk of recurrence by about 30% [Citation2]. Although the use of adjuvant endocrine therapy has clearly improved breast cancer survival, a significant number of endocrine-treated patients develop recurrent disease, 19.1% and 22.7% at 10-year follow-up after 5 years of aromatase inhibitor or tamoxifen, respectively [Citation2]. Therefore, a large number of ER-positive breast cancer patients will receive adjuvant chemotherapy before endocrine therapy and at present, we have no biomarker to select patients who will require chemotherapy or can be spared chemotherapy.
ER drives growth of breast tumors responding to endocrine therapy. ER becomes activated upon binding of estrogen and functions as a transcription factor, which regulates nearly a thousand genes [Citation3,Citation4]. Patients with as little as 1% ER-positive tumor cells derive benefit from endocrine therapy [Citation5], but superior benefit is observed in patients with tumors expressing higher level of ER [Citation5] and also in patients with tumors expressing the estrogen-inducible progesterone receptor (PR) [Citation6,Citation7]. In a pilot study with archival tumor tissue from a small subgroup of patients with advanced hormone receptor (ER and/or PR) positive breast cancer participating in the international PO25 clinical trial comparing first line letrozole to tamoxifen [Citation8], we have previously tested an ER activity profile consisting of ER and the three estrogen-stimulated proteins PR, Bcl-2 and IGF-IR [Citation9–12]. In the subgroup of patients treated with letrozole, we found significantly longer progression-free survival for patients with high ER activity profile compared with the other patients [Citation9].
The aim of the current study was to test whether the high ER activity profile was associated with longer disease-free and overall survival, and whether the profile was predictive of benefit from letrozole compared to tamoxifen.
Material and methods
Patients
Archival formalin-fixed and paraffin-embedded primary tumor tissues from Danish breast cancer patients who participated in the international randomized double-blinded clinical phase-III trial, BIG 1-98, were included in the study. In BIG 1-98, the efficacy of five years of tamoxifen or letrozole monotherapy, or their sequence was compared in postmenopausal women with hormone-receptor (ERα and/or PR)-positive breast cancer [Citation13–15]. In total, 1396 Danish patients, enrolled between 1998 and 2003, were randomized in BIG 1-98, and primary tumors from 1323 patients were available for tissue microarray (TMA) preparation as previously described [Citation16]. The biomarker study was conducted according to the Declaration of Helsinki and approved by The Danish National Committee on Biomedical Ethics in Denmark in 1997 (KF02 1 178/97), and an addendum approved in 2004 (KF 12 – 142/04). All patients had given written informed consent before inclusion in the BIG 1-98 study.
Immunohistochemical (IHC) analysis and evaluation
Immunohistochemical staining of ER, HER-2, PR, Bcl-2 and IGF-IR was performed as previously described [Citation9,Citation16]. The following primary antibodies were used: ER (clone ER1D5, 1:200; Immunotech, Trichem Aps, Frederikssund, Denmark), PR (clone 16, 1:200, Novocastra, Trichem Aps), IGF-IR (clone 24-31, 1:200, Thermo Fisher Scientific, Waltham, MA) and Bcl-2 (clone 124, 1:300, Dako Denmark A/S, Glostrup, Denmark). The specificity of the immunoreactions was verified by substitution of the primary antibody with the corresponding concentration of mouse IgG1, X0931, (Dako Denmark A/S). HER-2 IHC staining was performed with the antibody included in ‘HercepTest for the TechMate Instrument’ (Dako Denmark A/S) according to the manual. Tumors with IHC HercepTest score 2+ were further analyzed by fluorescence in situ hybridization (FISH) using Histology FISH accessory kit K5599 (Dako Denmark A/S), as recommended in the HercepTest Guidelines. Positive control slides with breast tumor tissue were included as controls in each run.
Semiquantitative determination of the IHC staining was performed without knowledge of the clinical data. Nuclear staining of ER and PR, and cytoplasmic staining of Bcl-2 was determined according to the method by Allred et al. [Citation17] as previously described [Citation16]. Membrane IHC staining of IGF-IR and HER-2 was scored according to the HercepTestTM guidelines [Citation16]. HER-2 gene amplification was determined by counting 60 HER-2 signals (red) and CEN17 (green) signals in nuclei from representative tumor areas and the HER-2/CEN17 ratio was calculated [Citation18]. A HER-2/CEN17 ratio ≥2 indicates a HER-2-amplified tumor, whereas an HER-2/CEN17 ratio <2 indicates a HER-2 nonamplified tumor, which is classified as HER-2-negative.
Statistical analysis
All clinical data were collected and monitored by the International Breast Cancer Study Group (IBSCG) and the Danish Breast Cancer Cooperative Group (DBCG). The statistical analyses were conducted at DBCG. Follow-up time was quantified in terms of a Kaplan–Meier estimate of potential follow-up. Kaplan–Meier plots were used to illustrate disease-free (DFS) and overall (OS) survival. As in the BIG 1-98 study, DFS was defined as the time from randomization to the earliest of any of the following events: recurrence of the disease at a local, regional or distant site; a new invasive cancer in the contralateral breast; new secondary nonbreast cancer; or death without a previous cancer event. Time-to-event outcomes, DFS and OS, were analyzed according to the intention-to-treat principle (ITT). Follow-up in the sequential arms was censored at 2 years, that is, at the time of scheduled cross over to enable comparison of pure tamoxifen- and letrozole-treated patients. The associations between ER profile and time-to-event endpoints, DFS and OS, were analyzed using the log-rank test stratified by treatment arm. Hazard ratio (HR) estimates were obtained from analysis using Cox proportional hazards models. Multivariate analyses included age, nodal status, tumor size, histological type and grade as covariates. Potential treatment effect heterogeneity according to ER profile and its component markers PR, Bcl-2 and IGF-IR was investigated by subgroup analysis and test for heterogeneity.
The number of deaths observed was compared with the number of deaths expected, calculated by applying age- and calendar year-specific female mortality figures of the general Danish population to the person years of risk. Standard mortality ratio (SMR) was computed as the ratio of the observed to the expected number of deaths and 95% confidence intervals (CI) were computed based on the assumption that the observed number of deaths followed a Poisson distribution. The SMR was analyzed using univariate Poisson regression model. All p values were two-tailed. Statistical analyses were performed with the SAS v9.4 program package (SAS Institute Inc., Cary, NC, USA).
Results
IHC staining and patient data
In this study, archival primary tumor tissues from 1323 of the 1396 Danish breast cancer patients enrolled in the BIG 1-98 study between 1998 and 2003 were included for IHC analyzes to detect the expression of ER, HER-2 and the ER-regulated proteins PR, Bcl-2 and IGF-IR. The staining was scored semiquantitatively either using the Allred method (ER, PR and Bcl-2) or the HercepTest score (HER-2, IGF-IR), as described in Material and Methods. The results from the scorings are shown in . Only 2% of the tumors were classified as ER negative (Allred scores 0 and 2). Intermediate (Allred scores 2–6) and high (Allred scores 7–8) ER levels were found in 26% and 68%, respectively (). A total of 116 patients were classified as HER-2 positive, of these 75 had HER2 score 3 and 41 had HER-2 score 2 and gene amplification. The nuclear expression of PR was negative/low in 19% of the tumors, whereas 39% of the tumors expressed intermediate and 37% high levels of PR. High cytoplasmic staining of Bcl-2 was seen in 60% of the tumors, whereas 30% expressed intermediate and 4% negative/low level of Bcl-2. Finally, the membrane expression of IGF-IR was high (HercepTest score 3) in 25% of the tumors, whereas 46% showed intermediate (HercepTest score 2) and 17% no or low (HercepTest scores 0–1) expression.
Table 1. IHC scoring results.
Standard clinicopathological parameters have previously been published [Citation19]. For analysis, a complete set of the investigated IHC markers were required. Patients with ER-negative tumors were excluded. Also patients with HER2-positive tumors were excluded as today, these patients will receive treatment with trastuzumab and chemotherapy before endocrine therapy. No statistical significant differences were observed in included (N = 969) and excluded (N = 354) patients in the prognostic variables; age, nodal status, tumor size, tumor grade and histological type. The number of patients randomized to letrozole or tamoxifen were well balanced, 489 (50.5%) and 480 (49.5%), respectively.
Marker evaluation of ER activity profiles
ER-positive and HER-2-negative patients were classified in ER activity profile groups based on the level of the IHC staining for PR, Bcl-2 and IGR-IR, . High ER activity profile was defined as high level of all three markers (102 patients), intermediate ER activity profile required 2 markers at high level and one at intermediate level (238 patients). Low ER activity profile consisted of tumors with only one marker at high level and tumors with at least one marker at intermediate level (627), whereas negative ER activity profile required negative values for all three markers (2), . The baseline characteristics for the patients in the different ER activity profile groups are shown in . The number of patients randomized to letrozole or tamoxifen was well balanced in all groups and the age distribution was similar among the groups. Statistically significant differences in nodal status, tumor size and grade (p = .003, .006 and .005, respectively), were observed between patients according to ER activity profile groups.
Table 2. Composition of ER activity profiles among 969 patients with ER-positive and HER-2-negative tumors.
Table 3. Baseline characteristics of ER activity profile groups among 969 patients with ER-positive and HER-2-negative tumors.
The Kaplan–Meier plots shown in revealed a significant association between the high ER activity profile and increased disease-free (p = .008) and overall (p = .01) survival, (). Noteworthy, only 16 of the 102 patients with high ER activity profile had recurred and nine had died. The univariate analysis comparing the 867 patients with intermediate or lower ER activity profile with the 102 patients in the high ER activity profile group revealed a significantly higher risk of relapse (HR = 2.00, CI = 1.20–3.32, p = .008) and death (HR = 2.33, CI = 1.19–4.56, p = .01), (). A comparison of the separate groups of intermediate and low ER activity profile groups with the high ER activity profile group as reference showed a statistically significant higher risk of relapse and death with lower ER activity profile (p = .0008 and p = .0003, respectively). In the multivariate analysis including the standard covariates; age, nodal status, tumor size, tumor grade and histological type, the ER activity profile was a significant and independent marker of prolonged disease free (p = .04) but not of overall survival (p = .06), ().
Figure 1. Kaplan–Meier survival curves demonstrating percentage DFS and OS for patients whose tumors have high ER activity profile versus other patients. p Values from log-rank testing.
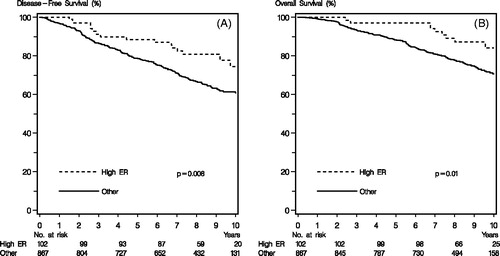
Table 4. Comparison of disease-free and overall survival in patients with high ER activity profile versus others and high ER activity profile versus intermediate and low ER activity profile.
No differences in DFS or OS were observed for tamoxifen- or letrozole-treated patients grouped according to ER activity profile, (). Also, the individual markers in the ER activity profile, PR, Bcl-2 and IGF-IR, did not disclose significant differences between tamoxifen- or letrozole-treated patients, ().
Table 5. Cox-regression analysis comparing disease-free and overall survival between letrozole and tamoxifen grouped by ER activity profile, PR, bcl-2 and IGF-IR.
The finding that only nine of the 102 patients had died during the estimated median potential observation time of 95.5 months prompted an exploratory analysis of the standardized mortality rate (SMR) of the high ER profile group versus an age-matched female population. The expected number of deaths is 6.6 in the age-matched female population, and the SMR is 1.36 (CI: 0.71–2.61). The SMR for the whole group of patients was 2.44 (CI: 2.08–2.86) with no statistically significant difference comparing SMR for patients with high ER activity profile and the remaining group, p = .07.
Discussion
Expression of ER in breast tumors is a prerequisite for estrogen-driven tumor growth, but not all patients with ER-positive tumors benefit from endocrine therapy, and therefore, adjuvant chemotherapy followed by adjuvant endocrine therapy is recommended for the majority of ER-positive breast cancer patients. Estrogen regulates the expression of a large number of genes and high expression of known estrogen-stimulated proteins like PR, Bcl-2 and IGF-IR in the tumor tissue reflects estrogen-driven tumor growth and may be better markers to select patients who may benefit from endocrine therapy than ER alone. In a previous small study with breast cancer patients receiving either tamoxifen or letrozole for advanced disease, we have found that letrozole-treated patients with a high ER activity profile consisting of high expression of PR, Bcl-2 and IGF-IR had significantly longer time to tumor progression [Citation9]. In this study including 1323 Danish patients from the BIG 1-98 study who received tamoxifen, letrozole or a sequence of the two drugs for early breast cancer, we found significantly improved DFS and OS in the 102 patients with a high ER activity profile compared to the remaining patients, p = .008 and p = .01, respectively. The multivariate analysis adjusted for the prognostic factors of age, lymph nodes, tumor size, grade and histological type disclosed that high ER activity profile was an independent marker for DFS (p = .04) and borderline for OS (p = .06).
In a recent study including 6529 postmenopausal ER-positive high-risk Danish breast cancer patients who had received only adjuvant endocrine therapy with 5 years of tamoxifen, an aromatase inhibitor or both in sequence, excess mortality compared to age-matched females was observed [Citation20], supporting that ER-positive high-risk breast cancer patients need additional treatment, for example, adjuvant chemotherapy. The standardized mortality rate for all 969 patients in this study is 2.44 (CI: 2.08–2.86), confirming the excess mortality in ER-positive high-risk patients receiving only endocrine therapy and supporting addition of adjuvant chemotherapy to the majority of these patients. In order to select patients who may be spared adjuvant chemotherapy, the number of deaths in the selected group must be similar to the number of deaths in an age-matched female population. Our finding of nine deaths was not significantly different from the expected 6.6. However, the ER activity profile group is too small to allow a final conclusion and the statistical analysis comparing SMR in the high ER activity profile group with the remaining patients did not reach statistical significance (p = .07). Our data support further analyses of the ER activity profile or other markers associated with estrogen-driven tumor growth as markers to select patients who may be spared adjuvant chemotherapy. Today, several gene expression-based predictors for breast cancer are available and most of these signatures identify a subset of luminal A patients with node-negative disease, who might be considered suitable for adjuvant endocrine therapy alone [Citation21]. A potential selection marker for adjuvant chemotherapy may be the 70-gene signature (MammaPrint), which divides breast cancer patients into high and low genomic risk groups for recurrent disease [Citation22]. Thus, the addition of the 70-gene signature to the high and low clinical risk groups defined by clinical–pathological factors disclosed that patients with high clinical risk of distant metastasis but low genomic risk had only slightly lower risk of distant metastasis with than without chemotherapy, indicating that these patients may not require chemotherapy [Citation23]. Of particular interest in relation to our ER activity profile is the EndoPredict® gene expression assay based on eight cancer-related genes covering biological processes such as proliferation, apoptosis, cell adhesion, cell signaling and ER expression [Citation24]. A low EndoPredict® risk score identifies patients who have significantly improved RFS at both 5 and 10 years of follow-up [Citation25]. An explorative analysis using the EndoPredict® assay showed that the expression levels of proliferative and ER-signaling genes contributed differentially to the prediction of early and late metastasis. Thus, high expression level of ER-signaling genes was significantly associated with reduced number of late metastases, whereas expression of proliferative-associated genes were significant for early (before 5 years) but not late metastases [Citation25]. These data are in good agreement with our data and support that determination of expression of estrogen-regulated genes may help selecting patients who may be spared adjuvant chemotherapy.
In the high ER activity profile group, we did not find differences in outcomes for patients treated with tamoxifen or with letrozole, indicating that both tamoxifen and letrozole are efficient therapy for these patients. This is in contrast to the results from our study of advanced breast cancer, which showed significantly improved progression-free survival and overall survival only in letrozole-treated patients with high ER activity profile [Citation9]. Although the number of patients receiving letrozole and tamoxifen was well balanced in the high ER activity profile group, the lack of a significant difference might be due to very few patients in each group or to the fact that in the BIG 1-98 study, patients allocated to tamoxifen monotherapy were allowed to cross over to treatment with letrozole, when it became evident that DFS with letrozole monotherapy was superior to tamoxifen [Citation13,Citation26]. The ability to cross over from tamoxifen to letrozole may also explain the lack of superiority of letrozole to tamoxifen in the whole group of Danish patients, ().
In summary, stratification of ER-positive, HER-2-negative Danish breast cancer patients from the BIG 1-98 study in ER activity profile groups disclosed significantly improved DFS and OS in the group with high profile. High ER activity profile is a marker for estrogen-driven tumor growth and these patients may have a better prognosis and/or particular benefit from adjuvant endocrine therapy. We suggest further analyses to test whether the high ER activity profile or other markers related to estrogen-driven tumor growth may be used to identify ER-positive high-risk breast cancer patients who may be spared adjuvant chemotherapy.
Acknowledgments
Annegrethe Schaadt and Vinni Bredahl are acknowledged for technical assistance. We kindly acknowledge the participating Danish Pathology Departments for supplying paraffin-embedded tumor material: Aalborg Syghus, Aarhus Hospital, Bispebjerg Hospital, Esbjerg Sygehus, Gentofte Hospital, Herlev Hospital, Hillerød Hospital, Hjørring Sygehus, Holstebro Sygehus, Hvidovre Hospital, Nykøbing Falster sygehus, Næstved Sygehus, Odense Hospital, Randers Sygehus, Rigshospitalet, Roskilde Sygehus, Skive Sygehus, Slagelse Sygehus, Svendborg Sygehus, Sønderborg Sygehus, Vejle Sygehus and Viborg Sygehus. We thank the International Breast Cancer Study Group for providing data on the Danish patients enrolled in the BIG 1-98 trial.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784.
- Early Breast Cancer Trialists’ Collaborative Group: Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of randomized trials. Lancet. 2015;386:1341–1352.
- Osborne CK, Schiff R. Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol. 2005;23:1616–1622.
- Frasor J, Danes JM, Komm B, et al. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinol. 2003;144:4562–4574.
- Viale G, Regan MM, Maiorano E, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole with tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. JCO. 2007;25:3846–3852.
- Osborne CK, Yochmowitz MG, Knight WA, III, et al. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980;46:2884–2888.
- Bardou VJ, Arpino G, Elledge RM, et al. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–1979.
- Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. JCO. 2003;21:2101–2109.
- Henriksen KL, Rasmussen BB, Lykkesfeldt AE, et al. An ER activity profile including ER, PR, Bcl-2 and IGF-IR may have potential as selection criterion for letrozole or tamoxifen treatment of patients with advanced breast cancer. Acta Oncol. 2009;48:522–531.
- Horwitz KB, Koseki Y, McGuire WL. Estrogen control of progesterone receptor in human breast cancer: role of estrogen and antiestrogen. Endocrinol. 1978;15:14–19.
- Dong LA, Wang WL, Wang F, et al. Mechanisms of transcriptional activation of bcl-2 gene expression by 17 beta-oestradiol in breast cancer cells. J Biol Chem. 1999;264:32099–32107.
- Stewart AJ, Johnson MD, May FE, et al. Role of insulin-like growth factors and the type I insulin-like growth factor receptor in estrogen-stimulated proliferation of human breast cancer cells. J Biol Chem. 1990;265:21172–21178.
- Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757.
- Mouridsen H, Giobbie-Hurder A, Goldhirsch A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:766–776.
- Regan MM, Neven P, Giobbie-Hurder A, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol. 2011;12:1101–1108.
- Henriksen KL, Rasmussen BB, Lykkesfeldt AE, et al. Semi-quantitative scoring of potentially predictive markers for endocrine treatment of breast cancer: a comparison between whole sections and tissue microarrays. J Clin Pathol. 2007;60:397–404.
- Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168.
- Olsen KE, Knudsen H, Rasmussen BB, Danish Breast Cancer Co-operative Group, et al. Amplification of HER2 and TOP2A and deletion of TOP2A genes in breast cancer investigated by new FISH probes. Acta Oncol. 2004;43:35–42.
- Larsen MS, Bjerre K, Lykkesfeldt AE, et al. Activated HER-receptors in predicting outcome of ER-positive breast cancer patients treated with adjuvant endocrine therapy. Breast. 2012;21:662–668.
- Ejlertsen B, Jensen MB, Mouridsen HT. Danish Breast Cancer Cooperative Group: Excess mortality in postmenopausal high-risk women who only receive adjuvant endocrine therapy for estrogen receptor positive breast cancer. Acta Oncol. 2014;53:174–185.
- Prat A, Parker JS, Fan C, et al. Concordance among gene expression-based predictors for ER-positive breast cancer treated with adjuvant tamoxifen. Ann. Oncol. 2012;23:2866–2873.
- Van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536.
- Cardoso F, Van’t Veer LJ, Bogaerts J, et al. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729.
- Filipits M, Rudas M, Jakesz R, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res. 2011;17:6012–6020.
- Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer. 2013;109:2959–2964.
- Giobbie-Hurder A, Price KN, Gelber RD. Design, conduct, and analyses of Breast International Group (BIG) 1-98: a randomized, double-blind, phase-III study comparing letrozole and tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive, early breast cancer. Clin Trials. 2009;6:272–287.
