Abstract
Background: The new American Joint Committee on Cancer eighth edition (AJCC8) staging is the first to describe separate clinical and pathology staging systems, but still has low performance to predict prognosis in patients with oesophageal/gastroesophageal junction (O/GOJ) adenocarcinoma, who are candidates for surgery. Recent studies have demonstrated that O/GOJ cancer patients with 18F-fluorodeoxyglucose (FDG) avid regional lymph nodes (RLNs) may have poor prognosis. The aim of our study was to examine whether the baseline assessment of the FDG uptake of RLN improves the prognostic accuracy of the new AJCC8 staging.
Patients and methods: This single-centre retrospective study included patients with operable FDG avid O/GOJ adenocarcinoma treated with perioperative chemotherapy. All patients were reclassified according to the new AJCC8 clinical staging. Prognostic factors for time-to-progression (TTP) and overall survival (OS) were explored.
Results: Of 430 patients included in the study, 180 (41.9%) had FDG avid RLN at baseline PET/CT scan before starting perioperative chemotherapy. The presence of FDG avid RLN was significantly and independently associated with shorter TTP and OS, especially in clinical stage III patients (p < .001 in both cases). Stage III patients with FDG avid RLN had similar TTP and OS to those with stage IVA. Classifying stage III patients with FDG avid RLN into stage IVA led to a significant improvement of the prognostic accuracy of the new AJCC8 clinical staging system (Harrell’s concordance index improved from 0.555 to 0.588, p < .001). Of 430 patients starting perioperative chemotherapy, 332 underwent radical tumour resection. The presence of FDG avid RLN before starting perioperative chemotherapy could additionally predict a significantly shorter postoperative time-to-relapse and OS (p < .001 in both cases).
Conclusions: We propose that the incorporation of RLN status (by FDG PET/CT scan) into the AJCC8 staging system of O/GOJ adenocarcinoma improves its prognostic accuracy and may also improve treatment stratification.
Introduction
Oesophageal/gastroesophageal junction (O/GOJ) adenocarcinoma has increased in incidence in recent decades in the Western World, in contrast to gastric cancer which has significantly reduced [Citation1], implying possible different pathogenetic pathways. Due to its high associated mortality rates there is an emphasis on early detection so that radical treatments can be administered to improve the 5 and 10-year mortality rates. In the UK, when the cancer is deemed to be localised by staging criteria, the treatment offered is radical surgery in conjunction with perioperative chemotherapy. The MAGIC trial [Citation2] showed that the addition of perioperative chemotherapy with epirubicin, cisplatin and 5-fluorouracil to radical surgery offered an absolute gain of 13% in five-year overall survival (OS). As a result, this regimen has been widely adopted in the UK as standard of care. However, only 70% of the patients initially deemed resectable within this study were radically operated on. Of these, the great majority of relapses occurred in the first two years postoperatively and only 34% remained disease-free after five years of follow-up [Citation2]. Another neoadjuvant treatment option is chemoradiotherapy [Citation3], which has been used less frequently in the past in UK, but currently is gaining more attention.
Of note, oesophagectomy is an operation with long postoperative hospitalisation and recovery periods, and significant morbidity and mortality rates [Citation4]. Similarly, chemotherapy given as an adjunct to surgery has additional significant associated toxicity [Citation3]. It is therefore imperative to accurately stage patients and avoid radical treatment that is likely to fail. Staging procedures as well as treatment concepts have significantly evolved in the previous two decades. However despite this, uncertainties continue to exist with respect to which patients will benefit from a radical approach and which will relapse. These staging procedures include computed tomography (CT) scan, endoscopic ultrasound (EUS) for primary tumour and regional lymph node (RLN) staging and fluorodeoxyglucose-positron emission/CT scan (FDG-PET/CT scan) [Citation3]. Recently, the eighth edition of the American Joint Committee on Cancer (AJCC8) introduced a new system for the clinical stage grouping (cTNM) of patients with O/GOJ adenocarcinoma, as defined by the above imaging methods [Citation5]. This clinical stage grouping was created with the aim to classify patients according to their prognosis before starting any treatment. However, its performance as a baseline prognostic tool still remains poor [Citation5]. Also, recent evidence showed that the FDG avidity of the RLN, rather than the primary tumour, might be positively correlated with poorer outcome [Citation6–9].
We conducted a study in O/GOJ patients starting standard perioperative chemotherapy with curative intent, with the primary aims of (1) confirming the prognostic accuracy of AJCC8 clinical staging and (2) assessing the baseline (pre-perioperative chemotherapy) prognostic significance of FDG avid RLN in patients with FDG avid O/GOJ adenocarcinoma. A secondary aim was to explore the association between the baseline FDG avidity and pathological status of RLN post perioperative chemotherapy.
Patients and methods
Patient population and management
In this retrospective cohort study, data from patients with FDG avid O/GOJ adenocarcinoma, who were deemed resectable according to local multidisciplinary team (MDT) decision, were collected and analysed. A primary tumour or a RLN was considered FDG avid when standardised uptake value max (SUVmax) was ≥3.0. Eligible patients had tumour epicentre located in the lower oesophagus or the GOJ (from 30 cm of the incisors until 2 cm beyond the junction). Decision on operability was based on eastern cooperative oncology group (ECOG) performance status (PS), co-morbidities, cardiopulmonary exercise (CPEX) score (≥15 mL/Kg/min) and clinical stage of the tumour. Clinical stages IIA–IIIC according to the AJCC seventh addition were defined as amenable to surgical resection. Staging workup included CT scan of the thorax, abdomen and pelvis, FDG-PET/CT scan, EUS and laparoscopy with peritoneal biopsies in case of tumours extending beyond the GOJ into the stomach. The final clinical stage was decided by a central upper gastrointestinal cancer MDT meeting. All patients were referred to one centre (Christie Hospital NHS Foundation Trust) and started neoadjuvant chemotherapy with curative intent between July 2009 and December 2015. The standard chemotherapy regimen used was ECX (epirubicin 50 mg/m2 on day 1, cisplatin 60 mg/m2 on day 1 and capecitabine 650 mg/m2 twice daily on days 1–21). In cases of clinically significant cardiac dysfunction, epirubicin was replaced by mitomycin (7 mg/m2 on alternate cycles), and in cases of glomerular filtration rate <40 mL/min cisplatin was replaced by carboplatin AUC 5 (area under the curve). After completing a maximum of three cycles of neoadjuvant chemotherapy, all patients underwent restaging CT scan, and optionally laparoscopy and EUS. If the tumour remained resectable, then they proceeded to radical operation, including Ivor-Lewis cardio-oesophagectomy or gastrectomy with transhiatal extension [Citation3]. Six to twelve weeks after operation (median, 9.7 weeks), all patients continued with the same regimen of adjuvant chemotherapy, if they were fit enough and had recovered from surgery. After a maximum of three cycles of chemotherapy, all patients were placed on active surveillance including 6-monthly clinical examination and imaging if indicated.
For the purpose of the analysis, all patients were retrospectively reclassified according to the AJCC8 into clinical stage groups. All procedures have been conducted in accordance with the Helsinki Declaration, as revised in 2013.
Statistical analysis
Patients were divided into FDG avid RLN vs FDG negative RLN. We tested the correlation between FDG avid RLN and baseline factors, including age, gender, ECOG PS, clinical stage, SUVmax of the primary tumour and location of the primary tumour, as well as the postoperative pathology status of RLN. Additionally, we examined the prognostic significance of the presence of FDG avid RLN. Outcome parameters included time-to-progression (TTP) and OS. TTP was defined as the time from the date of start of perioperative chemotherapy to the date of disease progression or relapse. Patients without evidence of disease progression or relapse were censored at the last date of follow up or at the date of death. OS was defined as the time from the date of start of perioperative chemotherapy to the date of death from any reason. Also, postoperative outcome parameters were time-to-relapse (TTR) and postoperative OS. Postoperative TTR was defined as the time from the date of radical surgery to the date of disease relapse. Patients without evidence of disease relapse were censored at the last date of follow up or at the date of death. Postoperative OS was defined as the time from the date of radical surgery to the date of death from any reason. The prognostic significance of baseline characteristics was tested by Cox proportional hazard models. Multivariable Cox-regression models analysed the prognostic significance of RLN FDG avidity against other baseline factors (age, gender, primary tumour location, clinical T (cT) stage, clinical N (cN) stage, primary tumour SUVmax), with a forward selection procedure and a selection criterion of p < .10. Internal validation was performed by bias-corrected and accelerated (BCa) bootstrap [Citation10]. Kaplan–Meier curves were constructed for different cN stages and FDG avidity of RLN and the difference in median survival was demonstrated by log-rank test. All comparisons were two-tailed with level of significance p < .05. Bonferroni correction for multiple testing was applied when required. The statistical analysis was performed using Statistical Package for the Social Sciences for Windows version 22 (SPSS, Inc., Chicago, IL, USA).
The primary endpoint of the study was the effect of baseline RLN status on OS. Secondary endpoints were the correlation between baseline RLN status and TTP or TTR and postneoadjuvant pathology stage.
Results
Basic characteristics and treatment
In total, 430 patients were included in the study; in 180 (41.9%) of them, baseline PET/CT scan demonstrated the presence of FDG avid RLN. Basic patient and tumour characteristics according to the presence or absence of FDG avid RLN are described in . Tumours with FDG avid RLN were associated with more advanced stage and poorer PS. Among them, 377 patients (87.7%) received three cycles of neoadjuvant chemotherapy, while the rest received 1–2 cycles. Also, 332 patients (77.2%) underwent radical surgery. Standard right-sided transthoracic cardio-oesophagectomy with two-field lymphadenectomy was performed in 301 patients (90.7%), while a small minority of 31 patients (9.3%) did not have thoracotomy, but underwent gastrectomy with transhiatal extension, because of contraindications for thoracotomy (N = 23; 6.9%) or because reassessment after neoadjuvant chemotherapy reclassified them as type III tumours (N = 8; 2.4%). Among 98 patients (22.8%) not having radical surgery, 65 (15.1%) were found to have unresectable disease at restaging CT scan or laparoscopy and 33 (7.7%) became inoperable due to toxicities from neoadjuvant chemotherapy or complications from cancer.
Table 1. Basic patient and tumour characteristics.
Prognostic significance of FDG avid RLN in association with AJCC8 clinical staging
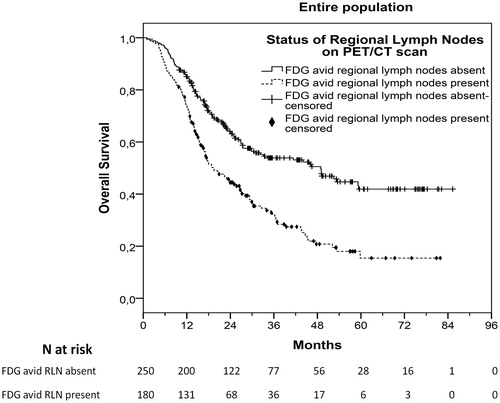
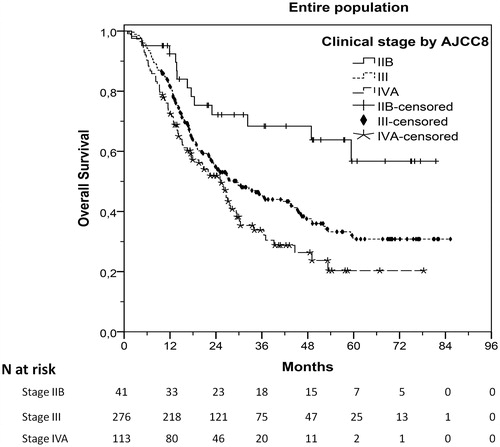
After a median follow-up of 43.2 months (range, 0.9–85.3), 221 patients (51.4%) had progressed and 238 (55.3%) had died. Median TTP was 20.1 months (95%CI 17.0–23.2) and median OS was 27.8 months (95%CI 22.4–33.2). AJCC8 clinical staging was significantly associated with TTP (stage III vs IIB, HR 2.23, 95%CI 1.24–4.03, p = .008; stage IVA vs IIB, HR 3.20, 95%CI 1.73–5.92, p < .001; stage IVA vs III, HR 1.43, 95%CI 1.07–1.91, p = .015) and OS (stage III vs IIB, HR 2.14, 95%CI 1.21–3.77, p = .009; stage IVA vs IIB, HR 2.89, 95%CI 1.60–5.23, p < .001; stage IVA vs III, HR 1.35, 95%CI 1.02–1.79, p = .035). Respective survival curves are shown in and S1. Additionally, patients with FDG avid RLN compared to those without FDG avid RLN had significantly shorter median TTP (p < .001) and OS (p < .001), as shown in Table S1 and in , S2, and Table S3.
Figure 1. Overall survival (OS) of patients with different clinical stages by AJCC8 (eighth edition of the American Joint Committee on Cancer staging).
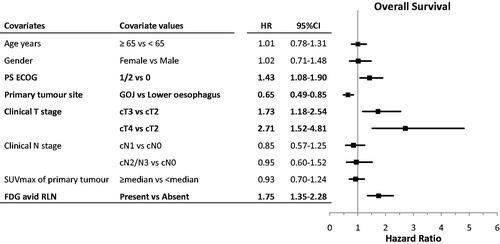
Other prognostic factors for TTP and OS are shown in Table S1 and and S3. The presence of FDG avid RLN remained an independent indicator of poor prognosis for TTP and OS in multivariable analysis ( and S3). Other independent prognostic factors were cT stage, ECOG PS for both PFS and OS and the site of the primary tumour only for OS.
Figure 3. Multivariable analysis of baseline prognostic factors for overall survival and respective forest plots. CI, confidence interval; cN, clinical N stage; cT, clinical T stage; ECOG, eastern cooperative oncology group; FDG, fluorodeoxyglucose; HR, hazard ratio; LNs, lymph nodes; PS, performance status; RLN, regional lymph nodes; SUVmax, maximum standardised uptake value.
Prognostic significance of FDG avidity of RLN on baseline PET/CT scan
Our analysis showed that FDG avid RLN was an independent poor prognostic factor. In addition, we examined the prognostic role of the status of RLN within clinical stages III and IVA by AJCC8, aiming to investigate whether PET/CT scan improves the prognostic accuracy of AJCC8 staging. Notably, in the subgroup of patients with clinical stage III disease patients with RLN staged as cN0 (cT3N0, cT4aN0) had similar TTP and OS with those staged cN1 (cT2N1, cT3N1, cT4aN1) (log-rank chi-square 1.39, p = .239 for TTP and log-rank chi-square 0.003, p = .953 for OS). Thus, patients with these stages were correctly included in the same stage by AJCC8 (stage III).
In contrast, the FDG uptake of RLN could successfully distinguish patients with different prognoses within clinical stage III by AJCC8. Patients with clinical stage III disease and FDG avid RLN had significantly shorter TTP and OS compared to those with clinical stage III and FDG negative RLN (log-rank chi-square 16.07, p < .001 for TTP, log-rank chi-square 12.69, p < .001 for OS). Furthermore, patients with clinical stage III cN1 disease (cT2N1, cT3N1, cT4aN1) and FDG avid RLN had significantly worse TTP and OS compared to those with clinical stage III cN1 disease (cT2N1, cT3N1, cT4aN1) but FDG negative RLN (log-rank chi-square 14.13, p < .001 for TTP, log-rank chi-square 15.24, p < .001 for OS). Therefore, in clinical stage III patients, the status of RLN by FDG uptake criteria was prognostically important, while their status by EUS or CT scan criteria (cN0 vs cN1) did not have any prognostic significance.
Although not significant, in the subgroup of patients with clinical stage IVA by AJCC8, the presence of FDG avid RLN was associated with a trend for significantly shorter OS (log-rank chi-square 3.59, p = .058), but not TTP (log-rank chi-square 0.30, p = .583) compared to those with FDG non-avid RLN.
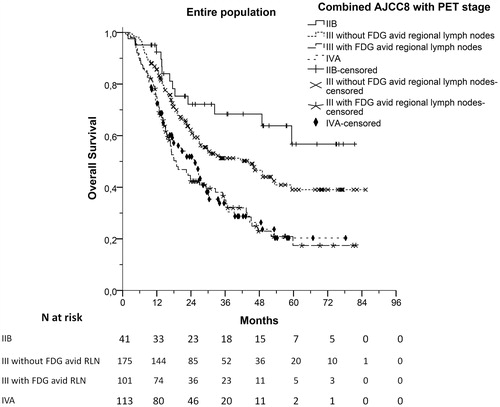
Based on our results we propose that clinical stage III could be divided according to FDG uptake of RLN. As shown in and S4, clinical stage III patients with FDG avid RLN had similar TTP and OS compared to those with stage IVA disease (log-rank test, p = .768 and p = .820, respectively). Thus, patients could be allocated into four different groups with distinct prognoses ( and S5). Patients with clinical stage IIB had the best prognosis, stage III patients with FDG negative RLN had intermediate prognosis, while both stage III patients with FDG avid RLN and stage IVA patients had the worst prognosis and could be classified in the same group. This classification led to a significantly improved prognostic accuracy compared to the new AJCC8 clinical staging system (c-index = 0.588 [95%CI 0.585–0.591] vs 0.555 [95%CI 0.552–0.558], respectively, p < .001). This prognostic model was internally validated by bootstrap analysis (Table S2).
Figure 4. Allocation of patients in four prognostically relevant groups for overall survival by combining the eighth edition of the American Joint Committee for Cancer (AJCC8) clinical staging and 18F-fluorodeoxyglucose (FDG) avidity of regional lymph nodes (RLN). CI, confidence intervals; HR, hazard ratio.
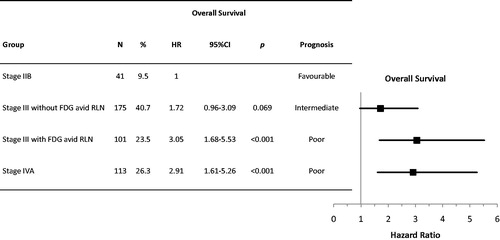
Figure 5. Overall survival (OS) of patients allocated in four different groups with distinct prognoses by combining clinical stage according to the eighth edition of the American Joint Committee for Cancer staging (AJCC8) and the presence or absence of FDG avid regional lymph nodes.
As shown in , the presence of FDG avid RLN was associated with higher primary tumour SUVmax. Further analysis with adjusted Cox-regression analysis for primary tumour SUVmax showed that the presence of FDG avid RLN was associated with poor TTP (HR 1.97, 95%CI 1.51–2.57, p < .001) and OS (HR 1.97, 95%CI 1.52–2.54, p < .001) independently to the primary tumour SUVmax.
Surgical pathology results and association between baseline clinical and post-neoadjuvant pathology stage
Surgical (post-neoadjuvant) pathology results of the 332 patients who underwent radical tumour resection are summarised in Table S3. Clinical and pathological N stages (cN and ypN) were compared for each patient. With regard to the comparison between cN and ypN stage, 114 patients (34.4%) were upstaged, 123 (37.0%) remained at the same stage and 95 (28.6%) were downstaged after completing the preoperative part of ECX-based chemotherapy.
We examined the correlation between cN stage and FDG uptake of RLN with ypN stage. Of 201 patients with infiltrated RLN (ypN+) at operation, 165 (82.1%) had been cN positive and 97 (48.3%) had been FDG avid at baseline. Among 131 patients with non-infiltrated RLN (ypN-), 52 (39.7%) had been cN0 and 99 (75.6%) had been FDG negative at baseline. Thus, cN stage was more sensitive and less specific in identifying infiltrated lymph nodes on pathology, while FDG-PET/CT RLN assessment was more specific but less sensitive.
Effect of the presence of FDG avid RLN at baseline on postoperative outcome
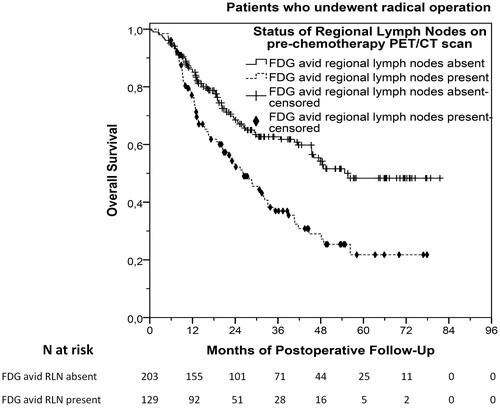
In this cohort, FDG-PET/CT scan was not repeated after preoperative chemotherapy as restaging exam before radical surgery. Hence, we attempted to correlate the results of baseline FDG-PET/CT scan that was performed before preoperative chemotherapy with postoperative TTR and OS. Among 332 patients who underwent radical operation, 150 (45.2%) relapsed and 154 (46.4%) died after a median postoperative follow-up period of 41.4 months (range, 0.4–81.5). Median postoperative TTR was 21.9 months (95%CI 17.6–26.1) and median postoperative OS was 40.7 months (95%CI 31.3–50.1). Patients with FDG avid RLN before starting preoperative chemotherapy (at baseline) demonstrated significantly shorter postoperative TTR (HR 2.03, 95%CI 1.47–2.80, p < .001) and OS (HR 1.92, 95%CI 1.40–2.64, p < .001). Median postoperative TTR was 13.5 (95%CI 9.8–17.3) and 33.6 months (95%CI 16.6–50.5) for patients with or without FDG avid RLN respectively (p < .001), while median postoperative OS was 26.2 (95%CI 19.6–32.8) and 54.7 months (95%CI not assessable) for patients with or without FDG avid RLN respectively (p < .001), as shown in and S6.
Discussion
Treatment of O/OGJ carcinomas is dictated by AJCC staging. Stage III carcinomas may be operable but five year survival outcomes are poor. Better stratification of disease subtypes will lead to improved survival outcomes and mitigate unnecessary treatment related toxicity.
FDG-PET/CT scan has been established as the main imaging method to exclude distant metastasis in patients with O/GOJ cancer. Furthermore, several investigators have observed a correlation between baseline tumour FDG-PET/CT characteristics and treatment outcome. Blom et al. [Citation6] showed that the metabolic tumour volume was weakly associated with better response to CRT. Hong et al. [Citation7] assessed diverse baseline FDG-PET parameters, such as SUVmax, peak SUV (representing the highest SUV in the locoregional area), total SUV (adding SUV of different sites in the locoregional area) and number of PET abnormalities (sum of all the FDG avid sites). Obviously, the number of PET abnormalities was proportional to the extent of lymph node metastasis in PET avid tumours. This last parameter, number of PET abnormalities, was found to have prognostic significance for DFS and OS in 47 patients with O/GOJ cancers of diverse histologic subtypes, receiving different chemotherapy regimens and approximately half of them having surgery [Citation7]. These findings were confirmed in a large cohort of patients with squamous cell carcinoma of the oesophagus who underwent preoperative chemotherapy; the number of FDG positive lymph nodes was prognostic [Citation8]. Moreover, Gillies et al. [Citation9] demonstrated the prognostic significance of the presence of FDG-positive lymph nodes in patients with O/GOJ adenocarcinoma who received preoperative chemotherapy.
Possible explanations for the above findings could be that FDG avidity might reflect the biological aggressiveness of the tumour and/or the extent of lymph node involvement. With regard to the first hypothesis, this has not been confirmed in the literature. Some reports have shown that patients with FDG avid primary tumours had a trend for poorer outcome compared to patients with non-avid lesions [Citation11]. However, other literature has found no association between FDG avidity and outcome [Citation7,Citation12,Citation13]. In addition, patients with tumours characterised by baseline SUVmax < 6 had similar survival when treated with preoperative or definitive CRT, implying that surgery might not be necessary in these cases and supporting the idea that they are a lower risk cohort [Citation14]. A confounding factor may be the fact that primary tumour FDG uptake appears to be proportional to its size as well as to T stage, which are well-known prognostic factors [Citation15]. In our cohort SUVmax of the primary tumour was not identified as a prognostic factor.
A second reason why FDG positive RLN might be strong predictors of prognosis maybe that FDG positive lymph nodes simply reflect the presence of more extensive lymph node involvement. EUS is recognised to be the optimal method to assess the size and number of affected lymph nodes, especially in PET-negative tumours [Citation16]. However, EUS is highly operator-dependent, limiting its ability to provide consistent information [Citation17]. In addition, not all patients are able to undergo EUS, because of bulky or stricturing tumours blocking the passage of the endoscope. Finally, EUS usually cannot assess the intra-abdominal lymph nodes. In contrast, FDG PET/CT scan images can be reviewed in a binary manner where the lymph nodes are either FDG positive or negative, thus providing more objective data to determine prognosis. A significant limitation in the application of FDG-PET/CT scan is that a minority of patients have FDG negative tumours, excluding it as an appropriate staging method for them.
In contrast to the above findings, most of the published literature agrees that the change in tumour FDG uptake following treatment might be more important than the baseline FDG uptake. Interestingly, it has been reported that the reduction in SUVmax that is achieved after neoadjuvant chemotherapy [Citation8,Citation18,Citation19], preoperative CRT [Citation20–23], or definitive CRT [Citation11] is associated with favourable pathologic tumour stage and/or patient outcome. This correlation has also been confirmed by two meta-analyses, one including 798 patients with GOJ tumours [Citation24] and another of 1544 patients with O/GOJ cancer [Citation25]. However, not all studies have managed to confirm this association [Citation13]. Also, a more homogeneous FDG tumour uptake after preoperative CRT was usually associated with a better pathologic response [Citation23], while a reduction in metabolic tumour volume after CRT was predictive of greater histological tumour regression and longer survival [Citation12]. Moreover, CRT followed by surgery was shown to be as equally effective as definitive CRT in patients with good PET response, while those with poor PET response had better outcome when surgery follows CRT [Citation12]. In contrast, recent studies showed that FDG uptake characteristics of the primary tumour post neoadjuvant CRT [Citation26,Citation27] and the RLN post neoadjuvant chemotherapy [Citation28] were not able to predict the postoperative histopathological tumour results. A considerable weakness of these studies is that many of them [Citation12,Citation14,Citation18–24] included patients with mixed characteristics, i.e., both squamous and adenocarcinoma histology and different treatments, making difficult to draw clinically meaningful conclusions.
The main disadvantage of using early treatment, mid treatment or post-treatment characteristics as prognostic markers is that they can only be used after the patient has already set out on a radical pathway and therefore may be exposed to unnecessarily aggressive treatments with additional morbidity/mortality risk. On the contrary, baseline prognostic factors might be able to identify patients with very poor prognosis who might be better considered for the palliative chemotherapy pathway and therefore avoid an unnecessary surgery or might be eligible for clinical trials. Our patients underwent an FDG-PET/CT scan only once, before starting preoperative chemotherapy. Thus, the post-chemotherapy FDG uptake of RLN was not assessed. Nevertheless, this study demonstrated that the presence of FDG avid RLN before starting chemotherapy was strongly associated with an adverse postoperative outcome. Therefore, repeating PET/CT scan post chemotherapy might not provide additional prognostic information and thus might not be necessary, considering the high cost.
Our study shows that the new AJCC8 clinical stage classification allocated the vast majority of cases in the intermediate prognosis group (clinical stage III), leaving about one quarter of the cohort in the poor outcome group (clinical stage IVA). FDG uptake of RLN combined with AJCC8 clinical staging led to the identification of a subgroup with poor prognosis within the stage III group. This subgroup had similar outcome with stage IVA patients. With this approach the adverse prognosis patients, i.e., stage III with FDG avid RLN plus stage IVA, made up approximately half of the full cohort. This reallocation substantially improved the prognostic accuracy of the model and could be considered for inclusion in the next AJCC edition. Similarly, patients with clinical stage IVA had a trend for even shorter OS, but not TTP, when their RLNs were FDG avid.
In conclusion, our study confirms the prognostic value of AJCC8 staging. Furthermore, we show for the first time that FDG avid RLN is independently associated with poorer TTP and OS, especially in patients with clinical stage III disease by AJCC8. Notably, these stage III patients with FDG avid RLN had inferior outcomes that were similar to patients with stage IVA disease. We propose that the incorporation of FDG PET/CT scan results regarding the status of RLN within the AJCC8 clinical staging system of O/GOJ adenocarcinoma may improve its prognostic accuracy and improve survival outcomes by improved treatment stratification.
IONC_A_1328127_Supplementary_Information.docx
Download MS Word (1.7 MB)Acknowledgments
G.P. and S.S. were granted a scholarship by the Hellenic Society of Medical Oncology.
Disclosure statement
Dr. Khoja is currently an employee of AstraZeneca Plc, but this work was done independently of AZ.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–527.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
- Rubenstein JH, Shaheen NJ. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology. 2015;149:302–317.
- Fischer C, Lingsma H, Hardwick R, et al. Risk adjustment models for short-term outcomes after surgical resection for oesophagogastric cancer. Br J Surg. 2016;103:105–116.
- Rice TW, Ishwaran H, Blackstone EH, et al. Recommendations for clinical staging (cTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:913–919.
- Blom RL, Steenbakkers IR, Lammering G, et al. PET/CT-based metabolic tumour volume for response prediction of neoadjuvant chemoradiotherapy in oesophageal carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:1500–1506.
- Hong D, Lunagomez S, Kim EE, et al. Value of baseline positron emission tomography for predicting overall survival in patient with nonmetastatic esophageal or gastroesophageal junction carcinoma. Cancer. 2005;104:1620–1626.
- Miyata H, Yamasaki M, Makino T, et al. Impact of number of [(18)F]fluorodeoxyglucose-PET-positive lymph nodes on survival of patients receiving neoadjuvant chemotherapy and surgery for oesophageal cancer. Br J Surg. 2016;103:97–104.
- Gillies RS, Middleton MR, Han C, et al. Role of positron emission tomography-computed tomography in predicting survival after neoadjuvant chemotherapy and surgery for oesophageal adenocarcinoma. Br J Surg. 2012;99:239–245.
- Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55–63.
- Suzuki A, Xiao L, Hayashi Y, et al. Prognostic significance of baseline positron emission tomography and importance of clinical complete response in patients with esophageal or gastroesophageal junction cancer treated with definitive chemoradiotherapy. Cancer. 2011;117:4823–4833.
- Jayachandran P, Pai RK, Quon A, et al. Postchemoradiotherapy positron emission tomography predicts pathologic response and survival in patients with esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:471–477.
- Elliott JA, O'farrell NJ, King S, et al. Value of CT-PET after neoadjuvant chemoradiation in the prediction of histological tumour regression, nodal status and survival in oesophageal adenocarcinoma. Br J Surg. 2014;101:1702–1711.
- Suzuki A, Xiao L, Taketa T, et al. Results of the baseline positron emission tomography can customize therapy of localized esophageal adenocarcinoma patients who achieve a clinical complete response after chemoradiation. Ann Oncol. 2013;24:2854–2859.
- Davies L, Mason JD, Roberts SA, et al. Prognostic significance of total disease length in esophageal cancer. Surg Endosc. 2012;26:2810–2816.
- Twine CP, Roberts SA, Rawlinson CE, et al. Prognostic significance of the endoscopic ultrasound defined lymph node metastasis count in esophageal cancer. Dis Esophagus. 2010;23:652–659.
- Lee WC, Lee TH, Jang JY, et al. Staging accuracy of endoscopic ultrasound performed by nonexpert endosonographers in patients with resectable esophageal squamous cell carcinoma: is it possible? Dis Esophagus. 2015;28:574–578.
- Miyata H, Yamasaki M, Takahashi T, et al. Determinants of response to neoadjuvant chemotherapy for esophageal cancer using 18F-fluorodeoxiglucose positron emission tomography (18F-FDG-PET). Ann Surg Oncol. 2014;21:575–582.
- Kauppi JT, Oksala N, Salo JA, et al. Locally advanced esophageal adenocarcinoma: response to neoadjuvant chemotherapy and survival predicted by ([18F])FDG-PET/CT. Acta Oncol. 2012;51:636–644.
- Kim SJ, Koo PJ, Chang S. Predictive value of repeated F-18 FDG PET/CT parameters changes during preoperative chemoradiotherapy to predict pathologic response and overall survival in locally advanced esophageal adenocarcinoma patients. Cancer Chemother Pharmacol. 2016;77:723–731.
- Swisher SG, Erasmus J, Maish M, et al. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776–1785.
- Ajani JA, Correa AM, Hofstetter WL, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2012;23:2638–2642.
- Tan S, Kligerman S, Chen W, et al. Spatial-temporal [18F]FDG-PET features for predicting pathologic response of esophageal cancer to neoadjuvant chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:1375–1382.
- Zhu W, Xing L, Yue J, et al. Prognostic significance of SUV on PET/CT in patients with localised oesophagogastric junction cancer receiving neoadjuvant chemotherapy/chemoradiation: a systematic review and meta-analysis. Br J Radiol. 2012;85:e694–e701.
- Schollaert P, Crott R, Bertrand C, et al. A systematic review of the predictive value of (18)FDG-PET in esophageal and esophagogastric junction cancer after neoadjuvant chemoradiation on the survival outcome stratification. J Gastrointest Surg. 2014;18:894–905.
- Heneghan HM, Donohoe C, Elliot J, et al. Can CT-PET and endoscopic assessment post-neoadjuvant chemoradiotherapy predict residual disease in esophageal cancer? Ann Surg. 2016;264:831–838.
- Arnett AL, Merrell KW, Macintosh EM, et al. Utility of (18)F-FDG PET for predicting histopathologic response in esophageal carcinoma following chemoradiation. J Thorac Oncol. 2017;12:121–128.
- Fencl P, Belohlavek O, Harustiak T, et al. FDG-PET/CT lymph node staging after neoadjuvant chemotherapy in patients with adenocarcinoma of the esophageal-gastric junction. Abdom Radiol (NY). 2016;41:2089–2094.