Abstract
Background: Miliary metastases are characterized by metastatic nodules that are diffuse, innumerable and small. The purpose of this study was to examine the incidence, prognostic significance and impact of epidermal growth factor receptor (EGFR) mutations for miliary metastases from non-small cell lung cancer (NSCLC).
Material and methods: Patients were identified from a Provincial cancer registry (British Columbia, Canada) for the period 2010–2012. Inclusion criteria were stage IV NSCLC at presentation and conclusive EGFR mutation testing. Miliary metastases were defined by subjective and objective radiographic criteria. The primary endpoint was the incidence of miliary lung, brain and liver metastases. Secondary endpoints were survival and the prognostic implication for each site of miliary metastases.
Results: For 543 patients, the total number of brain, lung and liver metastases were 165 (30.4%), 290 (53.4%) and 67 (12.3%), respectively. The EGFR mutation positive (EGFR+) subgroup had a significantly higher 3-year cumulative incidence of miliary brain (4.1 vs. 0.5%, p = .015) and miliary lung (11.6 vs. 3.3%, p < .001) metastases compared to EGFR wild type (WT). A greater proportion of metastases from EGFR + cancers were miliary for brain (8.5 vs. 1.7%, p = .035) and lung (18.9 vs. 6.9%, p = .003) sites. Only non-miliary brain (HR = 1.45) and liver (HR = 1.70) metastases predicted for poor overall survival.
Conclusions: Mutations in EGFR were associated with a higher rate of miliary brain and lung metastases. The presence of miliary metastases did not predict for poor overall survival.
Introduction
Miliary metastases describe a distinctive pattern of disease characterized by metastatic nodules that are diffuse, innumerable and small. This disease pattern is thought to arise from widely disseminated hematogenous spread. Miliary metastases have been associated with poor prognoses, although there is very little literature on comparative outcomes.
Miliary metastases from primary lung cancer are uncommon and the incidence is not known. Most published literature consists of case reports and there are no large studies or population data. Some case series have identified epidermal growth factor receptor (EGFR) mutations in lung cancer patients with miliary metastases [Citation1–3], suggesting an association. Larger studies are needed to support these anecdotes. This could have implications in anticipating disease behavior or in predicting the presence of driver mutations.
Mutations in the EGFR gene that result in constitutive activation of the tyrosine kinase are one of the most frequently identified driver mutations in non-small cell lung cancer (NSCLC) patients. Exon 19 deletions and exon 21 mutations account for 90% of these mutations. In NSCLC, EGFR mutations affect the biologic behavior of disease, as demonstrated by better survival and treatment response to EGFR tyrosine kinase inhibitors (TKI) [Citation4–6].
With institutional ethics approval, we examined the incidence of miliary lung, brain and liver metastases from advanced NSCLC. We also examined the association between EGFR mutations and miliary metastases and the impact of miliary metastases on survival.
Material and methods
Patient selection
We conducted a population-based, retrospective study of patients diagnosed with metastatic NSCLC for the years 2010–2012. Patients were identified from a Provincial Cancer Registry (British Columbia, Canada). The study inclusion criteria were non-squamous/non-neuroendocrine NSCLC, stage IV at initial diagnosis, and conclusive EGFR mutation testing. Individual medical records were used to obtain patient, disease and treatment characteristics. Diagnostic imaging reports were reviewed for lung, brain and liver metastases from the time of initial diagnosis to the time of death. Imaging studies identifying disease was also reviewed by a single observer oncologist. Diagnostic imaging was done at the discretion of the treating physician and as clinically indicated.
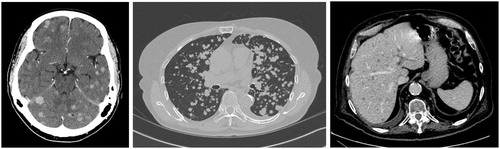
Metastases to the lung, liver and brain were classified as miliary vs. non-miliary at the time of first detection (). Metastases were miliary if they met both subjective and objective radiographic criteria. Subjectively, the metastases were diffuse, innumerable, and small. For the purpose of this study, miliary metastases were objectively defined as >15 metastatic nodules of sub-centimeter size (<1 cm diameter) and involving more than one organ lobe and bilaterally distributed. Patients with multiple pleural metastases, unilateral lymphangitic carcinomatosis and multifocal ground-glass opacities were excluded. When identifying metastases as miliary vs. non-miliary, the observer was blinded to the patient’s EGFR mutation status.
Mutation analysis
Patients were divided into EGFR mutation positive (EGFR+) and EGFR wild type (WT) subgroups. The EGFR mutation analysis was performed using DNA extracted from tumor tissue isolated from suitable blocks. Detection of EGFR exon 19 insertions/deletions and exon 21 L858R mutations were performed with a minimum of 400 ng of DNA by polymerase chain reaction using gene specific amplification primers, as previously described by Pan et al. [Citation7]
Study endpoints
The primary endpoint was the cumulative incidence of miliary metastases for lung, brain and liver sites. The secondary endpoints were survival and the prognostic implication of miliary metastases for each organ site. Overall survival was measured from the date of pathologic diagnosis to the date of death.
Statistical analysis
Patient and disease characteristics were compared between those with miliary metastases and those without using the Fisher Exact test for categorical variables and the Mann–Whitney U test for continuous variables. Cumulative incidence curves for miliary and non-miliary metastases were estimated for lung, brain and liver organ sites using the competing risks method. This method of analysis has significant advantages in providing a better estimation of incidence when there are high death rates from metastatic disease [Citation8,Citation9]. Patient death before the development of a site metastasis was considered a competing risk event. Patients who had not developed a site metastasis and had not died were censored at the time of last follow-up. Differences in the cumulative incidence curves between EGFR subgroups were assessed using Gray’s test. The association between miliary metastases, EGFR mutation status and patient characteristics were assessed using the Fine-Gray proportional sub-distribution hazards model. Survival was estimated using the Kaplan–Meier method and was compared using the log-rank test. The associations between survival, EGFR mutation status and miliary metastases were assessed using the Cox proportional hazards model. All reported p values are two-sided, with a p value <.05 set as the level of significance. Hazard ratios (HR) and confidence intervals (CI) were computed using Cox regression analysis. Analyses were performed using the R Statistical Language version 2.1.5 (Stanford University, Stanford, CA, USA).
Results
Patients
There were 1373 patients diagnosed with stage IV, non-squamous and non-neuroendocrine, NSCLC during the period of study. Five hundred forty three patients were eligible for study inclusion after excluding 746 patients without EGFR mutation testing and 84 with non-diagnostic results. For the study cohort, 121 (22.3%) patients had EGFR + cancers and 422 (77.7%) had EGFR WT. In the EGFR + subgroup, 73 (60%) cancers had exon 19 deletions and 48 (40%) had exon 21 L858R mutations. The median follow-up time for living patients was 34.9 months.
The median age, performance status and sex were not different between patients with and without miliary metastases (). For patients with miliary metastases of any site, the patients were different for EGFR mutation status, Asian ethnicity, and smoking status. By EGFR mutation status, a greater proportion of EGFR + vs. EGFR WT patients received TKI therapy (87 vs. 28%, p < .001). Chemotherapy use was not different between EGFR subgroups (49 vs. 49%, p = 1.0). Standard chemotherapy regimens used platinum doublets.
Table 1. Patient characteristics.
Incidence of miliary metastases
For the study cohort of 543 patients, 165 patients developed brain metastases. In six patients the brain metastases were miliary: two in the EGFR WT subgroup and four in the EGFR + subgroup (exon 19 = 4 vs. exon 21 = 0). For patients with brain metastases, the proportion that were miliary was larger in the EGFR + subgroup compared to EGFR WT (8.5 vs. 1.7%, p = .035). The 3-year cumulative incidence of miliary brain metastases for EGFR + patients was significantly higher than for EGFR WT (4.1 vs. 0.5%, p = .015) (). At presentation, two patients with miliary brain metastases were asymptomatic and four patients were symptomatic for headache and/or dizziness. None of the patients had focal or lateralizing neurologic signs or symptoms. Five of six patients with miliary brain metastases received whole brain radiotherapy. In four patients with EGFR + cancers, one had a complete radiographic response and three had a partial response. There was no follow-up brain imaging for the two EGFR WT patients, one of who had WBRT and the other who did not.
Table 2. Three-year metastases cumulative incidence rates.
A total of 290 patients had lung metastases. In 29 patients the lung metastases were miliary: 15 in the EGFR WT subgroup and 14 in the EGFR + subgroup (exon 19 = 8 vs. exon 21 = 6, p = .76). For patients with lung metastases, the EGFR + subgroup had a larger proportion that were miliary compared to EGFR WT (18.9 vs. 6.9%, p = .003). The 3-year cumulative incidence of miliary lung metastases for EGFR + patients was significantly higher than for EGFR WT (11.6 vs. 3.3%, p < .001) ().
A total of 67 patients had liver metastases. In two patients the liver metastases were miliary; both were EGFR WT. There was no significant difference in the proportion of patients with miliary liver metastases between EGFR subgroups (3.9 vs. 0%, p = .43). The 3-year cumulative incidence of miliary liver metastases for EGFR WT and EGFR + subgroups were 0.5 and 0% (p = .45), respectively ().
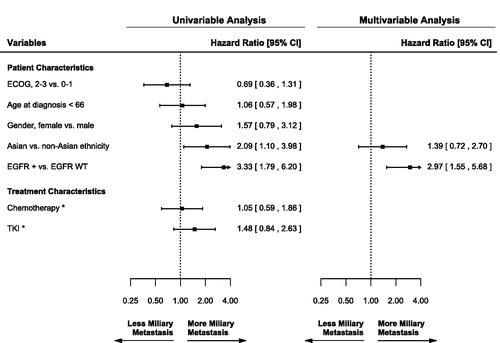
In univariable analysis, EGFR + mutation status and Asian ethnicity were significant factors for more miliary metastases of any organ site (). Age, Gender and systemic therapy use were not significant factors for the development of miliary metastases. Age <66 years was chosen as the cutoff because it was the median age of the study population. In multivariable analysis, only EGFR + mutation status was a significant factor for miliary metastases (HR = 2.97; CI = 1.55–5.68; p < .001).
Figure 2. Univariable and multivariable analysis for miliary metastases of any organ site. Inclusion for multivariable analysis was limited to variables significant in univariable analysis. *Analyzed as time-dependent variables. ECOG: Eastern Cooperative Oncology Group performance status; EGFR: epidermal growth factor receptor; +: mutation positive; WT: wild type; TKI: tyrosine kinase inhibitor.
Survival
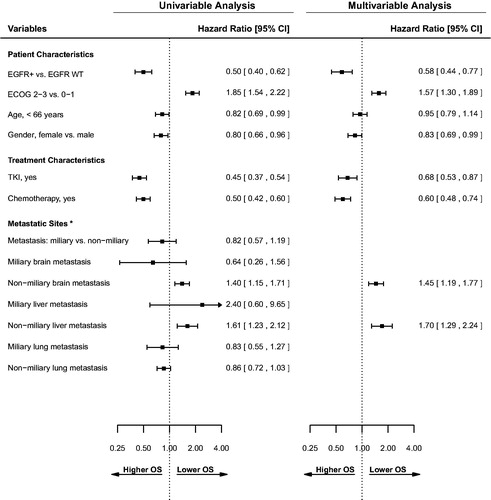
The univariable and multivariable Cox regression analysis for overall survival are presented in . In univariable analysis, EGFR + status, younger age (< 66 years), and female gender were significant factors for longer survival. Miliary metastasis to any organ site was not a significant factor for survival. In multivariable analysis, EGFR + status remained significant for longer survival (HR = 0.58; CI = 0.44–0.77; p < .001). Chemotherapy use (HR = 0.6; CI = 0.48–0.74; p < .001) and TKI use (HR = 0.68; CI = 0.53–0.87; p < .001) were treatment factors significant for longer survival in univariable and multivariable analysis.
Figure 3. Univariable and multivariable analysis for overall survival. Inclusion for multivariable analysis was limited to variables significant in univariable analysis. *All metastatic sites were analyzed as time-dependent variables. ECOG: Eastern Cooperative Oncology Group performance status; EGFR: epidermal growth factor receptor; +: mutation positive; WT: wild type; TKI: tyrosine kinase inhibitor.
For 165 patients with brain metastases, the median survival after brain metastases diagnosis was 6.5 months: 5.7 months for EGFR WT cancers vs. 10.4 months for EGFR + (p = .002). In univariable analysis, the presence of miliary brain metastases was not a significant factor for survival. For EGFR subgroups, there was no difference in the median survival after miliary vs. non-miliary brain metastases for EGFR + patients (12.4 vs. 9.0 months, p = .36) and for EGFR WT patients (4.6 vs. 5.7 months, p = 1.0).
For 290 patients with lung metastases, the median survival after lung metastases diagnosis was 11.9 months: 9.5 months for EGFR WT vs. 21.4 months for EGFR + (p = .001). In univariable analysis, the presence of miliary lung metastases was not a significant factor for survival. For EGFR subgroups, there was no difference in the median survival after miliary vs. non-miliary lung metastases for EGFR + patients (17.2 vs. 21.4 months, p = .63) and for EGFR WT patients (8.2 vs. 9.7 months, p = .36).
For 67 patients with liver metastases, the median survival after liver diagnosis was 4.3 months. The small incidence of miliary liver metastases did not lend itself to a detailed statistical evaluation.
Discussion
To our knowledge this is the only study examining the relationship between miliary lung, brain and liver metastases from NSCLC and EGFR mutations in a population-based cohort of patients with mixed ethnicity. We demonstrate a higher incidence of miliary lung and brain metastases in patients with the most common EGFR mutations, even when adjusted for survival. To take into account differences in death rates between EGFR + and EGFR WT subgroups, our analysis used a competing risk methodology with death as a competing event. Furthermore, the EGFR + subgroup had a larger proportion of lung and brain metastases that were miliary compared with non-miliary. In our analysis, patients with EGFR + cancers were three times more likely to present with miliary metastases.
Our literature search finds sparse case reports of EGFR mutations in patients with miliary lung metastases. Poonia et al. [Citation1] reported on a single patient with miliary lung metastases. Pathology showed adenocarcinoma with an in-frame deletion of exon 19. Laack et al. [Citation2] reported on five patients with a miliary pattern of pulmonary metastases, all who were never-smokers with adenocarcinoma histology and exon 19 deletions. A larger study by Wu et al. [Citation10] found that patients with miliary intrapulmonary carcinomatosis had higher rates of EGFR mutations than those without, 70 vs. 56%, specifically relating to exon 19. Our study did not find a difference between EGFR exon 19 and exon 21 subtypes for miliary lung metastases.
A separate case report by Mochizuki et al. [Citation3] describes an EGFR mutation in two patients with miliary brain metastases. In a study of 57 patients with NSCLC and brain metastases, Sekine et al. [Citation11] reported that patients with exon 19 deletions had more multiple and smaller brain metastases with less peritumoral edema. Similarly, Yuan et al. [Citation12] reported a trend toward multiple brain lesions in EGFR + patients who presented with late brain metastases. Our study supports an association between miliary brain metastases and exon 19 mutations.
Miliary liver metastases were rare. This could be, in part, because fewer liver metastases were detected overall. However, it may have been an underestimation, reflecting the difficulty in detecting small liver lesions radiographically. Certainly, this would be consistent with the findings in a report by Fazio et al. [Citation13] in which no imaging modality (magnetic resonance imaging (MRI) or computed tomography (CT) or somatostatin receptor scintigraphy) was able to detect miliary dissemination of neuroendocrine carcinoma in the liver at the time of surgery, thus underestimating liver disease burden.
The impact of other driver mutations on the development of miliary metastases is not known. We only examined EGFR mutations because of the available data at our institution. Several case reports suggest that this metastatic behavior may not be unique to EGFR mutants. Falk et al. [Citation14] described miliary brain and lung metastases in a single patient with an EML4-ALK gene translocation and no EGFR mutation. Dziadziuszko et al. [Citation15] reported a case of miliary brain metastases in a patient with ROS1 rearranged lung adenocarcinoma. Further studies of other driver mutations are needed. The cofounding influence of other driver mutations on our results is likely small because the frequencies of other driver mutations are low and multiple driver mutations concurrently in the same tumor are rare.
In the absence of a formal radiographic definition of ‘miliary’ metastases, we used subjective and objective measures. Our objective criteria specified a number and size to be applied across several organ sites for the purposes of this study. Our more generous number and size criteria were used because of the anticipated difficulty in resolving tiny lesions in the liver and brain with CT imaging, as many of the patients did not have MRI. In actuality, miliary metastases identified on our cohort were too numerous to count and much smaller than the <1 cm size criteria. A potential bias in our study is the absence of routine imaging for all patients. In our retrospective study, imaging was often symptom-driven, rather than routinely scheduled, as in a clinical trial. This probably caused us to underestimate the incidence of miliary metastases because macrometastases are more likely to cause symptoms than miliary metastases. Other limitations of our study include the small size of some subgroups, which reduced the power of some analyses. We did not find that miliary metastases were a significant factor for survival, but this could be because there were few patients with miliary metastases and the resulting large confidence intervals for survival.
With a better knowledge of how genetic mutations affect disease behavior in NSCLC, we may be able to better anticipate patterns of metastatic spread. Conversely, we may be able to use disease behavior to predict the presence of driver mutations. From our study, we put forth that miliary metastases are a credible characteristic in identifying patients who are more likely to carry an EGFR mutation. This may be useful for treatment decisions with an EGFR-TKI when genetic analysis is unavailable or non-diagnostic.
Conclusions
Mutations in EGFR are associated with a higher rate of miliary brain and lung metastases. Clinicians should be attentive to this pattern of presentation in patients with EGFR driver mutations. In contrast to prior reports, the presence of miliary metastases did not predict for poor overall survival, likely because miliary metastases were associated with the favorable prognosis subtype of EGFR + NSCLC.
Disclosure statement
The authors report no conflicts of interest.
References
- Poonia S, Berge EM, Aisner DL, et al. EGFR exon 19 deletion mutations and systemic/central nervous system miliary metastasis: clinical correlations and response to therapy. Clin Lung Cancer. 2014;15:387–389.
- Laack E, Simon R, Regier M, et al. Miliary never-smoking adenocarcinoma of the lung: strong association with epidermal growth factor receptor exon 19 deletion. J Thorac Oncol. 2011;6:199–202.
- Mochizuki S, Nishimura N, Inoue A, et al. Miliary brain metastases in 2 cases with advanced non-small cell lung cancer harboring EGFR mutation during gefitinib treatment. Respir Investig. 2012;50:117–121.
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139.
- Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol. 2010;28:744–752.
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29:2866.
- Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7:396–403.
- van Walraven C, McAlister FA. Competing risk bias was common in Kaplan–Meier risk estimates published in prominent medical journals. J Clin Epidemiol. 2015;69:170–173.
- Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430.
- Wu SG, Hu FC, Chang YL, et al. Frequent EGFR mutations in nonsmall cell lung cancer presenting with miliary intrapulmonary carcinomatosis. Eur Respir J. 2013;41:417–424.
- Sekine A, Kato T, Hagiwara E, et al. Metastatic brain tumors from non-small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer. 2012;77:64–69.
- Yuan R, Yamada A, Weber B, et al. Radiographic patterns and survival of patients with early and late brain metastases in EGFR wild type and mutant non-small cell lung cancer. J Neurooncol. 2016;127:525–533.
- Fazio N, Di Meglio G, Lorizzo K, et al. Miliary hepatic metastases from neuroendocrine carcinoma. Dig Surg. 2008;25:330.
- Falk AT, Poudenx M, Otto J, et al. Adenocarcinoma of the lung with miliary brain and pulmonary metastases with echinoderm microtubule-associated protein like 4-anaplastic lymphoma kinase translocation treated with crizotinib: a case report. Lung Cancer. 2012;78:282–284.
- Dziadziuszko K, Szurowska E, Pienkowska J, et al. Miliary brain metastases in a patient with ROS1-rearranged lung adenocarcinoma: a case report. J Thorac Oncol. 2014;9:e34–e36.