Abstract
Background: The introduction of adjuvant chemotherapy following surgery for early breast cancer (BC) and its integration into routine clinical practice has consistently improved clinical outcomes. Since the addition of other agents to the contemporary standard of care containing an anthracycline, cyclophosphamide and a taxane has not lead to further prolongation of survival, subsequent efforts concentrated on escalating the administered doses and reducing the time interval between chemotherapy cycles. These strategies have been extensively evaluated in randomized trials and dose dense chemotherapy is now recommended by clinical practice guidelines.
Method: Eligible trials were identified by searching the EMBASE, Pubmed, Scopus and Cochrane Library databases, as well as conference papers. The findings, shortcomings and impact of these studies are presented and critically discussed.
Results: Although a large number of randomized trials has established the value of adjuvant chemotherapy, important questions remain unanswered. Ongoing research focuses on omitting treatment in good risk patients, identifying patients most likely to benefit from a dose dense approach and on administering personalized doses such as in tailored dose chemotherapy.
Conclusions: Adjuvant chemotherapy for early BC is an evolving art. Further optimizations could potentially improve outcomes for a patient subset and spare others from unnecessary treatment-related toxicity.
Introduction
As early as in the turn of the previous century it had become apparent that patients with presumably completely resected early breast cancer (BC) could still relapse, develop clinically overt metastases and die of their disease. Increasingly radical attempts at surgical cure did not improve outcomes but instead caused significant morbidity [Citation1]. Thus, it was theorized that cancer cells dislodged at the time of surgery were responsible for subsequent relapses. Since then, a large number of randomized trials has been conducted, demonstrating the unequivocal impact of postoperative cytotoxic chemotherapy in prolonging survival, presumably due to the eradication of micrometastases. The results of these trials are summarized in the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis: adjuvant anthracyclines result in an absolute decrease of overall mortality of 5% compared to no chemotherapy [Relative Risk (RR) 0.84, 95% Confidence Interval (CI) 0.78–0.91]; the addition of taxanes further decreases overall mortality by 3% (RR 0.90, 95% CI 0.79–0.93) [Citation2].
Initially, chemotherapy was administered every 3–4 weeks in order to allow for marrow recovery. As supportive care was gradually improving, especially with the introduction of myeloid growth factors (granulocyte colony stimulating factor, GCSF), the administration of larger cumulative doses at shorter intervals without delays was made possible. Since then, non-hematologic adverse events such as mucositis, for which effective preventive measures are not commonly available [Citation3], have emerged as the dose limiting toxicities. The results of trials that pursued this strategy are herein reviewed, with the aim to demonstrate that despite the recent shift in attention towards the neoadjuvant setting, the science and art of ACT in early BC are still evolving, with novel strategies being incorporated in clinical practice.
Dose intense chemotherapy
The intensification of ACT (dose intense chemotherapy, DI-CT) constitutes the increase of administered doses, commonly measured by mg per m2 of body surface and divided by time, usually per week, to the maximum tolerated dose [Citation4]. Dose intensification is based on the hypothesis that drug resistance in cancer cells may be overcome by higher doses of certain chemotherapeutics which exhibit a linear dose–response curve [Citation5], such as the alkylating agents. Before the development of GCSF, the dose limiting toxicity was bone marrow failure. However, the introduction of myeloid growth factors permitted the administration of increased doses of certain drug classes [Citation6], hopefully leading to the eradication of minimal residual disease.
The results of selected trials of DI-CT are summarized in . One of the first large randomized trials that evaluated DI-CT in BC was conducted by the Cancer and Leukemia Group B (CALGB). One thousand five hundred and seventy two patients were randomized in three arms to receive cyclophosphamide (C) 600 mg/m2, doxorubicin (A) 60 mg/m2, and 5-fluorouracil (F) 600 mg/m2 (high dose CAF); C400A40F400 (moderate dose); or C300A30F300 (low dose), every 28 days. Both overall survival (OS) and disease free survival (DFS) were prolonged at the high and moderate dose group compared to the low dose group; outcomes were similar between the former two [Citation7]. Although initially hailed as a positive trial for DI-CT, according to contemporary standards the administered doses were inadequate and the results of this trial serve as an indicator that subtherapeutic doses lead to inferior outcomes. The French Adjuvant Study Group 05 compared two more intensive regimens, 5-fluorouracil 500 mg/m2, epirubicin (E) 50 mg/m2 and cyclophosphamide 500 mg/m2 (FE50C) versus the same regimen but with double the epirubicin dose (FE100C), both administered every 21 days (q3w). Results after 5 [Citation8] and 10 years [Citation9] of follow-up demonstrated the superiority of FE100C in terms of both OS and DFS. The results of this trial established FE100C as an option in early BC, albeit when compared to a suboptimal regimen according to modern practice.
Table 1. Adjuvant trials in early breast cancer evaluating dose intense chemotherapy.
On the other hand, at least three randomized trials, one conducted by CALBG (the 9344 study) and two by the National Surgical Adjuvant Breast and Bowel Project (NSABP B-22 and B-25) failed to confirm that dose escalation above a certain threshold escalation to all patients, regardless of the incurred toxicity, could further improve clinical outcomes [Citation10–12]. Moreover, high doses of cyclophosphamide were associated with an increased risk for delayed hematologic sequelae, such as acute myeloid leukemia (AML). In addition, attempts at escalating cumulative doses by administering further cycles of chemotherapy did not improve relapse free survival (RFS) or OS in a trial conducted by the CALGB [Citation13]. Further dose escalation with the administration of myeloablative doses of chemotherapy and rescue with stem cell transplantation has also been extensively evaluated. The results of 15 randomized trials were included in a meta-analysis, which concluded that OS is not prolonged with high dose ACT and autologous transplantation [Citation14]. Taken together, these data have disproven the notion that dose escalation alone, in the absence of predictive markers for better patient selection, is sufficiently effective to decrease post-surgery relapses and deaths due to BC.
Dose dense chemotherapy
Rationale
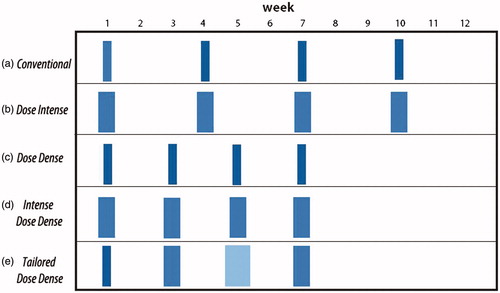
According to the Skipper–Schabel–Wilcox model, cell kill follows first order kinetics: tumor growth is exponential and cytotoxic chemotherapy removes a constant fraction of cells [Citation15]. Although this model formed the basis for the concepts of adjuvant chemotherapy and combinations of drugs, preclinical findings did not translate well to the clinical setting [Citation16]. In contrast, the Norton–Simon hypothesis dictates that the rate of cancer cell death as a result of cytotoxic treatment is directly proportional to the tumor growth rate at the time of its administration [Citation17]. Since Gompertzian kinetics apply, tumor cell growth will increase between CT cycles, meaning that reducing the meantime will result in more effective suppression of tumor regrowth and faster cell-kill [Citation16]. This premise functions as the rationale for the sequential administration of combinations of chemotherapeutics in a dose-dense manner (DD-CT; ), made possible by the introduction and widespread use of GCSF which allow the administration of multiple chemotherapy cycles in rapid succession, while retaining dose intensity.
Figure 1. Evolving strategies for adjuvant chemotherapy in breast cancer. In the conventional schedule (a), chemotherapy is administered every 21 days. In the dose-intense schedule (b), escalated doses are administered every 21 days. In the dose-dense schedule (c), conventional doses are administered every 14 days. In the intense dose-dense schedule (d), escalated doses are administered every 14 days. Finally, in the tailored-dose schedule (e), chemotherapy doses are determined by the hematologic nadirs and are administered every 14 days.
Dose dense versus conventional chemotherapy
The results of the trials that have compared DD-CT with conventional schedules are summarized in . The CALGB 9741 trial used a 2 × 2 factorial design and enrolled 2005 node-positive patients in order to address two issues: sequential versus concurrent administration of AC and the administration of AC and paclitaxel (P) q3w versus every 2 weeks (q2w) with growth factor support. DD-CT improved both DFS [Hazard Ratio (HR) = 0.74, p = .01] and OS (HR = 0.69, p = .013). Also, sequential chemotherapy was found to be equally effective with the concurrent administration [Citation18]. Similarly, the Italian GIM-II trial used a 2 × 2 design and again enrolled only patients with node-positive disease to receive EC with or without F and P either at two-week or at three-week intervals. The results of CALGB 9741 were confirmed, with DD-CT prolonging both DFS (HR = 0.78, 95% CI 0.65–0.94, p = .004) and OS (HR = 0.66, 95% CI 0.51–0.85, p = .001); the addition of F did not improve outcomes compared to EC [Citation19]. Importantly, in both these trials compliance and drug exposure was similar with the standard treatments due to the use of growth factors. With their results, these two trials have established the standard of care in early BC.
Table 2. Adjuvant trials in early breast cancer evaluating dose dense chemotherapy.
In contrast, several trials exploring the role of DD-CT have reported negative results. The prematurely closed due to poor accrual GONO-MIG (Gruppo Oncologico Nord-Ovest-Mammella Intergruppo) trial enrolled 1214 patients (64% node positive) to receive six cycles of F600E60C600 q2w (FEC-14) versus q3w (FEC-21). Although there were trends in recurrence (HR = 0.88, 95% CI 0.67–1.13, p = .22) and death (HR = 0.87, 95% CI 0.71–1.08, p = .29) favoring FEC-14, the differences were not statistically significant [Citation20]. Since this trial was underpowered to detect small differences in recurrence, its results are difficult to interpret. The second trial (UK TACT2) was reported in abstract form at the San Antonio Breast Cancer Symposium 2012 and was designed to address two issues: the role of increasing the dose density of epirubicin (q2w, E-14 versus q3w, E-21) and the role of adjuvant capecitabine following E when compared with CMF. At 5 years, both RFS (85.2% versus 86.4%, p = .60) and OS (89.3% versus 88.6%, p = .23) were similar between E-14 and E-21, respectively [Citation21]. The full publication of this trial is pending. The most recently published trial that compared DD-CT with conventional ACT was the NSABP B-38 which randomized 4894 node-positive patients in three groups: concurrent AC plus docetaxel (T; the TAC regimen) for six cycles; AC-14 → P-14; and AC-14 → P-14 with the addition of gemcitabine concurrently with paclitaxel. The addition of gemcitabine, which was based on the superiority of the combination versus paclitaxel monotherapy in advanced BC [Citation22], did not improve outcomes. Moreover, the DD AC → P regimen was not superior to TAC in terms of DFS (HR = 0.87, 95% CI 0.74–1.01, p = .07) and OS (HR = 1.01, 95% CI 0.82–1.23, p = .96) at 5 years [Citation23]. These results seem counter-intuitive, given the fact that TAC X 6 has been found to be of similar efficacy with a non-DD regimen (AC-21 → Docetaxel 100 mg/m2 q3w) at the BCIRG 005 trial [Citation24]; however, the different taxane used and the inherent hazards of performing cross trial comparisons should not alter the conclusion of the NSABP B-38 trial that six cycles of TAC is an effective adjuvant regimen. Finally, in a trial conducted by the Hellenic Cooperative Oncology Group (the HE 10/00 study), DD sequential E and P was compared with concurrent EP; the cumulative doses between these two groups were equal and both were followed by intensified CMF. Both DFS (74% versus 74%, p = .78) and OS (86% versus 85%, p = .45) were equivalent at five years [Citation25]. The non-conventional treatment regimens and the relatively small sample size (1121 patients) do not permit the generalization of these findings.
Indirect evidence in support of DD-CT is offered by recently published trials that compared the TC (docetaxel and cyclophosphamide q3w) regimen to anthracycline-containing ones. TC was established as an effective option at the US Oncology 9735 study; when compared to AC-21 it resulted in improved DFS and OS [Citation26]. In the 2016 annual meeting of the American Society of Clinical Oncology (ASCO), interim results of the Adjuvant Breast Cancer (ABC) suite of trials, which compared six cycles of TC to various DD and non-DD anthracycline-based regimens, were presented. The invasive DFS rates at 4 years were 88.2% and 90.7% for the TC and anthracycline-based groups, respectively (HR = 1.23, 95% CI 1.01–1.50, p = .04) [Citation27]. This finding of the non-inferiority of TC was shared by a smaller trial conducted by the Hellenic Oncology Research Group [Citation28]. Although it is unclear whether these results are owed to the omission of anthracyclines or to the DD regimens, they propose DD-CT as the treatment of choice when compared to yet another contemporary standard of care, the TC regimen.
Although the results of the reported randomized trials are conflicting, a meta-analysis which was updated in 2015 and included 17,188 patients from eight trials corroborate the initial findings of the CALGB 9741 trial: DD-CT resulted in improved OS [HR = 0.86, 95% CI 0.79–0.93, p < .0001] and DFS (HR = 0.84, 95% CI 0.77–0.91, p < .0001) when compared to conventional schedules [Citation29]. These findings support the use of DD-CT; however, it should be noted that this meta-analysis was not based on the analysis of individual patient data.
Combining dose dense and dose intense chemotherapy
Attempts at combining the escalation of both the administrated dose and the treatment density (intense, dose dense chemotherapy; IDD-CT) have been undertaken in four randomized trials with somewhat conflicting results, which are presented in . The first trial was conducted by the Eastern Cooperative Oncology Group (ECOG). In the E1199 trial, 4950 patients with node-positive or high risk node-negative BC received four cycles of AC-21 and were then randomized to receive either P or T, weekly or q3w. At the initial publication, weekly P 80 mg/m2 resulted in improvements in both DFS [Odds Ratio (OR) = 1.27, 95% CI 1.03–1.57, p = .006] and OS (OR = 1.32, 95% CI 1.02–1.72, p = .01) when compared to P 175 mg/m2 q3w. Moreover, T 100 mg/m2 q3w improved DFS when compared to P q3w (OR = 1.23, 95% CI 1.00–1.52, p = .02) but not OS (OR = 1.13, 95% CI 0.88–1.46, p = .25). Since weekly P leads to higher cumulative doses (240 mg/m2 at 3 weeks) and with increased density, the initial results indicated the superiority of an IDD-CT approach [Citation30]. These results have been updated with long-term follow-up: the difference in OS between weekly and q3w P was no longer statistically significant (HR = 0.87, 95% CI 0.75–1.02, p = .09). Interestingly, in an exploratory analysis, weekly P improved OS in triple negative BC (TNBC) (HR = 0.69, 95% CI 0.52–0.91, p = .019); in the hormone receptor (ER) positive, human epidermal growth factor receptor 2 (HER2) negative group, only T q3w prolonged DFS (HR = 0.76, 95% CI 0.63–0.91, p = .004) when compared with P q3w [Citation31].
Table 3. Adjuvant trials in early breast cancer evaluating dose intense/dose dense chemotherapy.
Following up on a previous trial by the European Organization for Research and Treatment of Cancer (EORTC) where CEF (oral C, E60 and F500 at days 1 and 8, every 28 days) was found to be non-inferior compared to E120C830 q2w (EC-14) [Citation32], the MA.21 trial enrolled 2104 patients with early node positive or high risk node negative BC to receive CEF X 6, EC-14 followed by P175 q3w or AC → P q3w (as in the CALGB 9741). The latter resulted in significantly inferior DFS compared to the former two regimens; no difference was detected between CEF and EC → P [Citation33]. The short follow-up period of 30 months is a confounding factor and long term results of this trial are awaited. Nevertheless, again an IDD regimen was superior to a conventional, albeit older and not up to contemporary standards, combination.
In a trial of IDD-CT by the Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) group, 1284 patients with four or more involved axillary lymph nodes were randomized to receive either E90C600 → P175 q3w or an IDD schedule of E150, P225, C2500 sequentially q2w, each for three cycles. The 5-year OS rates were 82% and 77% for the IDD and conventional regimens, respectively (p = .0285). However, the IDD schedule was associated with significant toxicities, both hematologic and non-hematologic. Importantly, there were four occurrences of AML or myelodysplastic syndrome at the IDD group versus none at the standard group [Citation34]. Whether this regimen is superior to established DD ones is currently unknown: the GAIN-1 trial from the same group, reported similar 5-year DFS and OS between this regimen and EC-14 followed by weekly P 67.5 mg/m2 plus capecitabine 2000 mg/m2 days 1–14, repeated q3w [Citation35].
Tailored dose chemotherapy
The doses of chemotherapy agents that are used in early BC are usually calculated in relation to the body surface area, a rather rudimentary approach that does not account for the significant interpatient variability in terms of the pharmacokinetic and pharmacodynamics properties of these agents [Citation36,Citation37]. As a result, meaningful and easily reproducible surrogate markers for the drug levels achieved are needed. Such a marker is the depth of cytopenias after each cycle of chemotherapy. Although five retrospective studies of nearly 2000 patients have established that failure to achieve hematologic toxicity is associated with inferior clinical outcomes when receiving conventional ACT [Citation38–42], routine clinical practice only requires reductions of doses in patients experiencing hematologic adverse events but not dose increases in those who do not. Therefore, patients could be both undertreated thus impairing survival and overtreated thus exposing them to unnecessary toxicity. Conceivably, adapting the administered doses according to prespecified algorithms based on hematologic toxicity could represent a major step towards the delivery of precision medicine.
This hypothesis was tested on the recently published PANTHER trial which was conducted by the Swedish Breast Cancer Group, the German Breast Cancer Group and the Austrian Breast and Colorectal Cancer Study Group. In total, 2017 patients were randomized to receive either FE100C q3w followed by T100 q3w or a tailored dose dense (TDD) regimen of EC → T, depending on hematologic nadirs. Of note, the FEC → T regimen which was established from the French PACS 01 trial is considered adequate treatment according to contemporary standards [Citation43]. Moreover, uniquely among all the previously mentioned studies, HER2 positive patients were routinely enrolled and treated with the standard of care 1 year of trastuzumab. These two relative strengths of the study mean that its results could be readily translated to routine practice.
Tailoring resulted in higher cumulative doses of E (median, 405 mg/m2 versus 300 mg/m2), C (median, 3306 mg/m2 versus 1500 mg/m2) and T (median, 334 mg/m2 versus 300 mg/m2) with the same therapy duration. The primary endpoint, which was 5-year RFS, bordered statistical significance but did not cross it (88.7% versus 85%, HR = 0.79, 95% CI 0.61–1.01, p = .062). Event free survival favored the experimental group (86.7% versus 82.1%, HR = 0.79, 95% CI 0.63–0.99, p = .042) and there was a trend towards improved OS (92.1% versus 90.2%, HR = 0.77, 95% CI 0.57–1.05, p = .091) as well. Although grade 3–4 hematologic and non-hematologic toxicities favored the FEC regimen, standard T resulted in increased hematologic adverse events compared to TDD, although it should be noted that patients at the experimental group did receive two more courses of chemotherapy compared with the standard group. There were no toxic deaths in the experimental group [Citation44]. These results demonstrate the feasibility of this adaptive approach, as the next logical step in the evolution of ACT. Longer follow-up is required in the Panther study, however, the present outcome data reveal clear trends in favor of TDD, which should be contemplated in particular for patients with high risk features.
Synthesis of the evidence and future perspectives
Interpreting the results of published trials of DI and DD-CT is an arduous task. The patients enrolled in each study differed greatly in terms of clinically relevant demographic (age) and pathologic (ER and HER2 status, node positivity) characteristics. Furthermore, the heterogeneity of the regimens that were evaluated does not permit cross-trial comparisons. Nevertheless, the results of CALGB 9741 and the GIM-II trials and of the meta-analysis by Petrelli et al., as well as the previously described weaknesses of the trials that did not report superiority for DD-CT, lead to the conclusion that AC or EC q2w followed by P q2w or weekly is a reasonable ACT regimen for early BC. Still, which is the taxane of choice is a matter of open debate. Although the E1199 trial showed that weekly P was not superior to T q3w, the previously mentioned exploratory analysis from this study possibly delineates the specific patient subset for each regimen. In addition, the S0221 trial conducted by the Southwest Oncology Group (SWOG) demonstrated that P q2w may be superior to weekly P, at least for patients with ER-positive and HER2-negative early BC [Citation45]. Further investigations are warranted in order to clarify this issue.
In the DD-CT trials, myeloid growth factor support was mandatory in order to retain dose intensity and avoid treatment delays that would hamper treatment efficacy. As a result, rates of neutropenia and febrile neutropenia are consistently lower compared to the standard treatment groups. Nevertheless, taking into account the potential for increased rates of other adverse events from DD-CT, particularly anemia, thrombocytopenia, mucositis and long term sequelae like myelodysplasia and acute leukemia [Citation29], better selection of patients is an urgent, albeit unmet need. In order to optimize treatment algorithms at the adjuvant setting, three questions need to be answered: which patients need ACT? which patients will benefit from DD-CT? and, finally, should doses be individualized?
Question 1: Do all patients with early breast cancer need adjuvant chemotherapy?
The proportional reduction of relapse and death caused by ACT is the same regardless of ER status according to the EBCTCG meta-analysis [Citation2]. Moreover, although the induction of amenorrhea has been linked with further improvement of outcomes [Citation46], the benefit to premenopausal ER-positive patients is not exclusively derived from the induction of amenorrhea caused by ACT, since chemoendocrine combinations lead to superior outcomes compared to endocrine therapy alone [Citation2]. Keeping in mind several limitations of the chemotherapy versus no chemotherapy trials included in the EBCTCG analysis (these trials are now over 30 years old, none of them included a taxane, doses and supportive care were suboptimal compared to contemporary standards and in half the studies no endocrine therapy was administered), no clinicopathologic subgroup seems to not benefit from chemotherapy. In addition, the magnitude of benefit expressed in the proportional reduction of risk of death, an important endpoint when exploring the effect of a treatment in the mortality of a subgroup of patients [Citation47], is similar regardless of clinicopathologic factors. As a result, high-level evidence derived from the patient level meta-analysis dictate that all patients derive the same relative benefit from ACT, although the absolute gains in risk reduction may be small in patients at low risk for relapse, thus allowing the omission of ACT according to contemporary clinical practice guidelines [Citation48].
Recent data indicate that potentially a subset of patients with ER-positive BC has an excellent prognosis with adjuvant endocrine therapy alone, since the absolute increase of OS induced by ACT is marginal [Citation2]. Sparing patients from the unnecessary short and long term toxicities of ACT can be accomplished with the aid of an abundance of gene signatures that have been endorsed by ASCO [Citation49]. The best validated is the Recurrence Score (RS), with two prospective trials underscoring the excellent prognosis of patients with ER-positive, node negative BC with RS ≤ 10 (TAILORx) and ER-positive, pN0-1 BC with RS ≤ 11 (German PlanB) [Citation50,Citation51]; lower cutoffs were used in these trials compared to earlier studies in order to minimize any potential undertreatment [Citation52]. The ongoing RxPONDER trial will help guide treatment decision for node-positive patients. It should be noted that the follow-up of these studies is relatively short for a disease such as ER-positive BC which has a prolonged natural history (69 months for TAILORx and 35 months for PlanB). Another gene signature, the Predictor Analysis of Microarray 50 (PAM50) has been shown to robustly predict the intrinsic subtype of the tumor [Citation53] and it can also generate the Risk of Recurrence (ROR) score which has been shown to predict 10-year RFS rates regardless of nodal status [Citation54]. Another prospectively evaluated gene profile is the Amsterdam 70-gene signature. In the MINDACT trial, patients with low nodal burden deemed to be high risk based on clinical factors and low risk based on the gene signature had a distant DFS of over 94% at 5 years without receiving chemotherapy, 1.5% lower than the patients that received chemotherapy. Similar trends in DFS (2.8% difference) and OS (1.4% difference) also favored the administration of chemotherapy in this critical patient subgroup, although the trial was not powered to perform these comparisons [Citation55]. While more mature data are awaited, using these gene signatures or others such as EndoPredict [Citation56] as guidance, carefully selected patients could be spared from ACT altogether and the population enrolled in trials evaluating DD-CT could be enriched with patients more likely to benefit from such treatment. However, large scale comparisons of these signatures have not been undertaken and it is thus unclear which one is the optimal for further development and use in clinical practice.
Question 2: Which patients benefit the most from a dose dense approach?
Attempts at identifying patients most likely to benefit from DD-CT have met with modest success, at best. In the aforementioned literature based meta-analysis conducted by Petrelli et al., the prolongation of OS only applied to ER-negative and not to ER-positive patients (HR = 0.8, p = .002 and HR = 0.93, p = .25, respectively) [Citation29]. Confusingly, in three randomized trials, the German AGO, the Italian GIM-2 and the PANTHER trial which was not included in the meta-analysis, an OS benefit was demonstrated in ER-positive patients [Citation19,Citation34,Citation44]. These results are in line with the findings of the EBCTCG meta-analysis regarding the benefit of ACT in ER-positive patients [Citation2]. Conversely, corroborating the results of the meta-analysis by Petrelli et al. are the findings of the E1199 and the CALGB 9741 trials [Citation18,Citation30]. Thus, it seems that although ER status could aid at the selection of candidates for DD-CT, ER-positivity does not preclude benefit. Whether the intrinsic subtype could further refine the selection of patients, given the known link between certain subtypes and chemosensitivity in BC, remains to be seen [Citation57].
Nodal stage has been found to correlate with both local and distant relapse, as well as death from BC [Citation58]. Regarding the predictive power of the number of positive lymph nodes, a retrospective analysis has demonstrated a significant prolongation of survival in patients with ≥11 positive lymph nodes receiving ACT [Citation59]. Thus, it seems logical that a more aggressive approach should be reserved for patients at the highest end of the risk spectrum. This matter has not been prospectively addressed and the inclusion of node positivity as an eligibility criterion does not result in a survival benefit from DD-CT, as demonstrated from the NSABP B-38 trial [Citation23]. In contrast, nodal burden seems to more accurately select appropriate candidates: the higher median number of positive lymph nodes at the AGO (eight positive nodes) and GIM-2 (five positive nodes) trials could potentially explain the discrepancies in reported results compared with the negative TACT 2 (one positive node) and NSABP B-38 (two positive nodes) trials [Citation60]. This analysis is purely exploratory and should not presently guide management decisions.
The previously described gene signatures have been shown to accurately predict lack of benefit from ACT in patients at low risk for recurrence. Conceivably, patients at the highest risk would need a more aggressive treatment approach. Although prospective data are lacking, retrospective analyses from observational or randomized studies could serve as a proof of principle.
On the other hand, owing to the conflicting results of reported trials, to the commonly observed increased toxicity and to the high costs of the supportive care (for example, myeloid growth factors) needed when administering DD-CT, support for its use is not unanimous. In the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015, a slim majority acknowledged the existence of specific high risk subgroups among ER-positive patients warranting the administration of DD-CT, while for triple negative disease the panel was divided regarding its use [Citation61]. Local practices and patient and physician preferences impact heavily on the choice of ACT regimens. The publication of patient-level meta-analyses on DD-CT may shed some light on this matter and help guide management algorithms.
Question 3: Is the individualization of administered doses a feasible strategy?
As described in the tailored dose chemotherapy section of this review and prospectively shown by the PANTHER trial, individualizing the administered doses based on a simple, reproducible and widely used biomarker such as the hematologic nadirs is a feasible strategy. Long term results from this study are needed in order to examine whether the strong trends for improved DFS and OS cross statistical significance and also to assess the long term toxicities of this schedule. Building up on previously acquired experience, the GAIN-2 study conducted by the German Breast Group is currently enrolling patients (clinicaltrials.gov identifier NCT01690702). Its aim is to compare the regimen from the PANTHER study to a modified version of the AGO IDD-CT regimen, where paclitaxel has been substituted with nab-paclitaxel and the dose of C has been lowered to 2000 mg/m2 due to concerns for delayed hematologic sequelae. The results of this trial will hopefully demonstrate whether an IDD or a tailored dose approach is optimal for patients at high risk for relapse (four or more involved nodes or HER2-positive or TNBC regardless of nodal status or luminal B subtype with involved nodes).
Conclusions
Following the success of the CALGB 9741 trial, DD-CT has become the standard option for patients with early BC deemed to be in high risk for post-surgical relapse. Many questions remain unanswered, such as the appropriate selection of patients, the regimen of choice and the integration of other agents at the adjuvant setting for specific subgroups, such as carboplatin for TNBC, anti-HER2 agents for HER-2 positive BC and more effective endocrine manipulation strategies for ER-positive BC. In addition, the inherent difficulty in conducting large adjuvant trials in BC which enroll thousands of patients for a multi-year follow-up and the gradual shift towards smaller neoadjuvant trials in molecularly defined populations with pathologic complete response serving as the primary endpoint, mean that many of these questions may never be answered. Nevertheless, a large body of literature supports the use of DD-CT in appropriately selected, high risk early BC; translational studies as part of prospective randomized trials are of paramount importance in order to shed some light in the underlying complexity of this patient population.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Halsted WS. I. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;46:1–19.
- Early Breast Cancer Trialists’ Collaborative G, Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444.
- Vadhan-Raj S, Goldberg JD, Perales MA, et al. Clinical applications of palifermin: amelioration of oral mucositis and other potential indications. J Cell Mol Med. 2013;17:1371–1384.
- Hryniuk W, Levine MN. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. J Clin Oncol. 1986;4:1162–1170.
- Von Hoff DD, Clark GM, Weiss GR, et al. Use of in vitro dose response effects to select antineoplastics for high-dose or regional administration regimens. J Clin Oncol. 1986;4:1827–1834.
- Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–170.
- Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998;90:1205–1211.
- French Adjuvant Study G. Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. JCO. 2001;19:602–611.
- Bonneterre J, Roche H, Kerbrat P, et al. Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French Adjuvant Study Group 05 randomized trial. JCO. 2005;23:2686–2693.
- Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983.
- Fisher B, Anderson S, Wickerham DL, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-22. JCO. 1997;15:1858–1869.
- Fisher B, Anderson S, DeCillis A, et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-25. JCO. 1999;17:3374–3388.
- Shulman LN, Cirrincione CT, Berry DA, et al. Six cycles of doxorubicin and cyclophosphamide or Paclitaxel are not superior to four cycles as adjuvant chemotherapy for breast cancer in women with zero to three positive axillary nodes: Cancer and Leukemia Group B 40101. JCO. 2012;30:4071–4076.
- Berry DA, Ueno NT, Johnson MM, et al. High-dose chemotherapy with autologous stem-cell support as adjuvant therapy in breast cancer: overview of 15 randomized trials. J Clin Oncol. 2011;29:3214–3223.
- Skipper HE, Schabel FM, Jr, Wilcox WS. Experimental evaluation of potential anticancer agents. Xiii. On the criteria and kinetics associated with “curability” of experimental leukemia. Cancer Chemother Rep. 1964;35:1–111.
- Norton L. Cancer log-kill revisited. Am Soc Clin Oncol Educ Book. 2014;3–7.
- Simon R, Norton L. The Norton–Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat Clin Pract Oncol. 2006;3:406–407.
- Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. JCO. 2003;21:1431–1439.
- Del Mastro L, De Placido S, Bruzzi P, et al. Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised phase 3 trial. Lancet. 2015;385:1863–1872.
- Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst. 2005;97:1724–1733.
- Cameron D, Barrett-Lee P, Canney P, et al. The UK TACT2 Trial: comparison of standard vs accelerated epirubicin in patients requiring chemotherapy for early breast cancer (EBC) (CRUK/05/019). San Antonio Breast Cancer Symposium; 2012; San Antonio, TX.
- Albain KS, Nag SM, Calderillo-Ruiz G, et al. Gemcitabine plus Paclitaxel versus Paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. JCO. 2008;26:3950–3957.
- Swain SM, Tang G, Geyer CE Jr, et al. Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: the NSABP B-38 trial. JCO. 2013;31:3197–3204.
- Eiermann W, Pienkowski T, Crown J, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with human epidermal growth factor receptor 2-normal, node-positive breast cancer: BCIRG-005 trial. JCO. 2011;29:3877–3884.
- Gogas H, Dafni U, Karina M, et al. Postoperative dose-dense sequential versus concomitant administration of epirubicin and paclitaxel in patients with node-positive breast cancer: 5-year results of the Hellenic Cooperative Oncology Group HE 10/00 phase III Trial. Breast Cancer Res Treat. 2012;132:609–619.
- Jones S, Holmes FA, O'shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183.
- Blum JL, Flynn P, Yothers G, et al. Interim joint analysis of the ABC (anthracyclines in early breast cancer) phase III trials (USOR 06-090, NSABP B-46I/USOR 07132, NSABP B-49 [NRG Oncology]) comparing docetaxel + cyclophosphamide (TC) v anthracycline/taxane-based chemotherapy regimens (TaxAC) in women with high-risk, HER2-negative breast cancer. American Society of Clinical Oncology Annual Meeting; 2016; Chicago, IL.
- Mavroudis D, Matikas A, Malamos N, et al. Dose-dense FEC followed by Docetaxel versus Docetaxel plus Cyclophosphamide as adjuvant chemotherapy in women with HER2-negative, axillary lymph node-positive early breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2016;27:1873–1878.
- Petrelli F, Cabiddu M, Coinu A, et al. Adjuvant dose-dense chemotherapy in breast cancer: a systematic review and meta-analysis of randomized trials. Breast Cancer Res Treat. 2015;151:251–259.
- Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671.
- Sparano JA, Zhao F, Martino S, et al. Long-term follow-up of the E1199 phase III trial evaluating the role of taxane and schedule in operable breast cancer. J Clin Oncol. 2015;33:2353–2360.
- Therasse P, Mauriac L, Welnicka-Jaskiewicz M, et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study. JCO. 2003;21:843–850.
- Burnell M, Levine MN, Chapman JA, et al. Cyclophosphamide, epirubicin, and Fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by Paclitaxel versus Doxorubicin and cyclophosphamide followed by Paclitaxel in node-positive or high-risk node-negative breast cancer. J Clin Oncol. 2010;28:77–82.
- Moebus V, Jackisch C, Lueck HJ, et al. Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III study. JCO. 2010;28:2874–2880.
- Moebus V, von Minckwitz G, Jackisch C, et al. German Adjuvant Intergroup Node-positive Study (GAIN): a phase III Trial Comparing Two Dose-Dense Regimens (iddEPC vs. ddEC-PwX) in high-risk early breast cancer patients. Ann Oncol. 2017 [cited Apr 28]. DOI:10.1093/annonc/mdx203.
- Gurney HP, Ackland S, Gebski V, et al. Factors affecting epirubicin pharmacokinetics and toxicity: evidence against using body-surface area for dose calculation. JCO. 1998;16:2299–2304.
- Sandstrom M, Freijs A, Larsson R, et al. Lack of relationship between systemic exposure for the component drug of the fluorouracil, epirubicin, and 4-hydroxycyclophosphamide regimen in breast cancer patients. JCO. 1996;14:1581–1588.
- Saarto T, Blomqvist C, Rissanen P, et al. Haematological toxicity: a marker of adjuvant chemotherapy efficacy in stage II and III breast cancer. Br J Cancer. 1997;75:301–305.
- Colleoni M, Price K, Castiglione-Gertsch M, et al. Dose-response effect of adjuvant cyclophosphamide, methotrexate, 5-fluorouracil (CMF) in node-positive breast cancer. International Breast. Cancer Study Group. Eur J Cancer. 1998;34:1693–1700.
- Poikonen P, Saarto T, Lundin J, et al. Leucocyte nadir as a marker for chemotherapy efficacy in node-positive breast cancer treated with adjuvant CMF. Br J Cancer. 1999;80:1763–1766.
- Cameron DA, Massie C, Kerr G, et al. Moderate neutropenia with adjuvant CMF confers improved survival in early breast cancer. Br J Cancer. 2003;89:1837–1842.
- Paridaens R, Wildiers J, Dumez H, et al. Impact of dose-intensity of adjuvant CMF on disease-free (DFS) and overall survival (OS) in breast cancer (BC): a retrospective analysis. 27th ESMO Congress; 2002 Oct 18–22; Nice: Annals of Oncology, Oxford University Press.
- Roche H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. JCO. 2006;24:5664–5671.
- Foukakis T, Von Minckwitz G, Bengtsson N, et al. Effect of tailored dose-dense chemotherapy vs standard 3-weekly adjuvant chemotherapy on recurrence-free survival among women with high-risk early breast cancer. JAMA. 2016;316:1888–1896.
- Budd GT, Barlow WE, Moore HC, et al. SWOG S0221: a phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer. JCO. 2015;33:58–64.
- Swain SM, Jeong JH, Geyer CE, Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–2065.
- Peto R. Current misconception 3: that subgroup-specific trial mortality results often provide a good basis for individualising patient care. Br J Cancer. 2011;104:1057–1058.
- Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v8–30.
- Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. JCO. 2016;34:1134–1150.
- Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014.
- Gluz O, Nitz UA, Christgen M, et al. West German Study Group Phase III PlanB Trial: first prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. JCO. 2016;34:2341–2349.
- Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26:721–728.
- Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167.
- Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–345.
- Cardoso F, Van't Veer LJ, Bogaerts J, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729.
- Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2– breast cancer patients. Br J Cancer. 2013;109:2959–2964.
- Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334.
- Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187.
- Schwentner L, Wockel A, Konig J, et al. Assessing the impact of CMF-like/anthracycline-based/anthracycline-taxane-based/dose-dense chemotherapy in dependency of positive axillary lymph nodes/hormone receptor-status/grading/T-stage on survival – a retrospective multi-centre cohort study of 3677 patients receiving adjuvant chemotherapy. Eur J Cancer. 2014;50:2905–2915.
- Mobus V. Adjuvant dose-dense chemotherapy in breast cancer: standard of care in high-risk patients. Breast Care (Basel). 2016;11:8–12.
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies – improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–1546.
