Abstract
Background: A named patient program (NPP) was designed to provide patients with advanced soft-tissue sarcoma (aSTS) access to pazopanib, a multitargeted tyrosine kinase inhibitor. The SPIRE study was a retrospective chart review of participating patients.
Patients and methods: Eligibility criteria for the NPP and SPIRE mirrored those of the pivotal phase-III study, PALETTE, which compared pazopanib with placebo in patients ≥18 years with aSTS and whose disease had progressed during or following prior chemotherapy or were otherwise unsuitable for chemotherapy. Outcomes of interest included treatment patterns, treatment duration, relative dose intensity, progression-free survival (PFS), overall survival (OS), clinical benefit rate, adverse events (AEs) and reasons for treatment discontinuation.
Results: A total of 211 patients were enrolled (median age 56 years; 60% female). Most patients received pazopanib in second- and third-line therapy (28.0% and 28.4%, respectively), followed by fourth line (19.0%) and ≥ fifth line (18.5%). The median duration of pazopanib treatment was 3.1 months (95% CI: 2.8–3.8), with a mean daily dose of 715 mg equating to 92% of recommended dose. Median OS was 11.1 months and clinical benefit rate was 46%. There was evidence of some clinical benefit across most histological subtypes. At study end, 40% of patients were alive and of these, 18% remained on pazopanib. Thirteen percent (13%) of patients discontinued pazopanib due to AEs.
Conclusions: The SPIRE study demonstrated activity of pazopanib in heavily pretreated aSTS patients in a compassionate use setting. No new safety concerns were noted. Reassuringly, the relative dose intensity of pazopanib was 92%.
Introduction
Sarcomas are a rare group of solid tumors and over 80% of sarcomas are soft-tissue sarcomas (STS) [Citation1,2]. STS include heterogeneous tumors, with over 40 malignant histological subtypes (defined by the current version of the World Health Organization’s histopathological classification) which can occur throughout the body [Citation2–4]. The heterogeneity of STS makes treating this disease a particular challenge. Prognostic factors for survival include: histological subtype and tumor site; tumor size; grade; age; performance status, and the presence of nodal or distant metastases [Citation3,Citation5]. Based on these factors, treatment options may include surgery, radiotherapy and/or chemotherapy [Citation6].
In locally advanced or metastatic disease, systemic therapy plays an important role [Citation5,Citation6]. Initial chemotherapy usually consists of an anthracycline with or without ifosfamide [Citation5,Citation6]. Treatment options in second line and beyond can vary according to histological subtypes and there is no standard chemotherapy in second line and beyond for patients who have progressed after an anthracycline-based treatment [Citation5–7].
Pazopanib (Votrient®) has been approved for pretreated advanced STS (aSTS) in the USA, Europe and in Japan and is indicated for the treatment of adult patients with selective subtypes of aSTS who have received prior chemotherapy for metastatic disease or who have progressed within 12 months after receiving adjuvant or neoadjuvant therapy. The recommended starting dose of pazopanib is 800 mg once daily [Citation8].
Pazopanib is an oral anticancer agent and a multitargeted tyrosine kinase inhibitor, that targets VEGFR1,2,3, PDGFR alpha and beta and KIT, thereby blocking tumor growth and inhibiting angiogenesis [Citation5,Citation8–13]. Pazopanib has demonstrated activity in patients with aSTS following prior therapy [Citation10,Citation11]. In the phase-III PALETTE study, pazopanib demonstrated a significant advantage in median PFS compared with placebo, 4.6 months versus 1.6 months respectively (hazard ratio [HR]: 0.31, 95% CI: 0.24–0.40; p < .0001) [Citation11]. Median OS was 12.5 months with pazopanib versus 10.7 months with placebo (HR: 0.86, 95% CI: 0.67–1.11; p = .25) [Citation11]. Pazopanib was well tolerated, with the most common grade 3/4 adverse events being fatigue (13.7%), increased alanine transaminase (ALT) (9.6%) and lymphopenia (9.6%) [Citation14].
This pazopanib NPP was launched during the pazopanib regulatory review process, upon request by physicians following the positive results of the PALETTE trial, to provide pazopanib treatment to appropriate patients with aSTS [Citation13]. The results obtained from the SPIRE study (Sarcoma named PatIent progRamme chart rEview) are described here.
Methods
This was a multicountry, multicenter, retrospective chart review study of treatment patterns and clinical outcomes in a subset of patients with aSTS who received pazopanib as part of the NPP. Prior to study conduct, ethics approvals were obtained in line with country-specific national regulations (further details provided in Supplementary section 1). Supplementary Figure 1 shows an overview of the patient identification, sampling and subject enrollment processes.
The pazopanib NPP included patients who were 18 years or older, with aSTS, with an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, with adequate organ system function and clinically normal cardiac function and had received prior systemic chemotherapy therapy for advanced disease unless it was considered unsuitable. Patients could not be treated concurrently with another anticancer therapy. Patients with STS histologies excluded from the PALETTE study were ineligible. Using a convenience sample of centers with higher numbers of NPP patients, all available patients from these study centers were included in the SPIRE study.
The primary objectives were to describe the number and type of prior and subsequent systemic treatments given to patients with aSTS who received pazopanib and to determine pazopanib treatment duration. Secondary objectives included relative dose intensity, dose modifications and reason for dose modifications, treatment discontinuation and reason for discontinuations for patients. Other outcomes of interest included PFS, OS, clinical benefit rate (complete response [CR] + partial response [PR] + stable disease [SD] + patients receiving pazopanib for ≥12 weeks with unknown/undocumented benefit), AEs (measured by Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 or 4.0), and reasons for treatment discontinuation. PFS and OS were estimated by the Kaplan–Meier method. The analysis was mainly descriptive with appropriate statistical methods, further details are provided in the Supplementary section 1.
Results
The data were collected from patient medical charts for patients who received pazopanib between 26th August 2011 and 1st August 2013. The study was conducted at 24 sites across six countries (Australia, Greece, Israel, Italy, the Netherlands and the United Kingdom). The median study period from pazopanib initiation to date of death or date patient was last known to be alive was 6.9 months. The mean time from first diagnosis of aSTS until initiation of pazopanib therapy was 27.3 months.
Patient characteristics
Data were collected on 211 patients out of a total of 531 patients with aSTS who received at least one dose of pazopanib in the NPP. Supplementary Table 1 shows baseline patient characteristics where the median age was 56 years, all patients had stage IV (i.e., metastatic) disease, and the majority of patients had lung metastases (74%, 155/211).
The largest subset of histological subtypes was leiomyosarcoma (LMS) (41%, 87/211) which included uterine LMS, nonuterine LMS and LMS of unknown location. The second and third largest histological subtypes were synovial sarcoma (11%, 24/211) and undifferentiated sarcoma (9%, 19/211).
Treatment patterns and pazopanib treatment
Overall in the total observed population of 211 patients, 6% of patients (13/211) received pazopanib as a first-line therapy, while 28% (59/211), 28% (60/211), 19% (41/211) and 19% (39/211) received pazopanib as a second-, third-, fourth- and fifth-line or above therapy on the NPP, respectively. The frequencies of treatment, including pazopanib and other systemic therapies, by the line of therapy, are described in . Thirty-nine percent (39%) of patients (83/211) received trabectedin either before or after pazopanib, 66% (55/83) of which had leiomyosarcoma.
Figure 1. Treatment regimens by line of therapy (observed population). *There are 67 patients who had 5th line or above which =129 regimens.
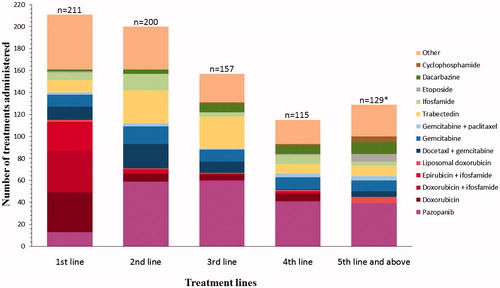
The mean daily dose of pazopanib was 715 mg, which translates to a mean relative dose intensity of 92% (). The mean starting daily dose was 738 mg, the majority of patients (179, 84.8%) started at a daily dose of 800 mg with 24 patients (11.4%) starting at daily doses ≤50% of the labeled dose of 800 mg/day. Twenty-two percent (47/211) of patients had at least one dose reduction and 20% (42/211) of patients experienced at least one dose interruption. The reasons for either a dose reduction or interruption were: non-serious AEs (65%, 58/89); serious AEs (14%, 12/89); patient’s decision unrelated to AE (10%, 9/89) and other (11%, 10/89). The main AEs leading to dose reductions included hypertension (3%, 6/211), diarrhea and nausea (2%, 5/211 each) and fatigue (1%, 3/211).
Table 1. Pazopanib average daily dose and relative dose intensity.
At the end of the study, 40% (84/211) of patients were still alive and of those patients still alive, 18% (15/84) remained on pazopanib. Reasons for pazopanib discontinuation are shown in Supplementary Figure 2, with the majority of patients (67%, 140/211) discontinuing due to disease progression.
The median treatment duration for pazopanib was 3.1 months (95% CI: 2.8–3.8) (). There was considerable variation in treatment duration by histological subtype, with some patients remaining on therapy for an extended duration and other patients coming off therapy quickly ( and Supplementary Figure 2), although some of the histological subtypes had small numbers of patients. However, it was notable that some patients across most histological subtypes stayed on therapy for an extended duration. The histological subtypes with the longest median treatment duration included: PEComa (8.2 months); aggressive fibromatosis (8.0 months); alveolar soft part sarcoma (7.1 months); desmoplastic small round cell tumor (5.7 months) and synovial sarcoma (5.1 months). Treatment duration also varied by ECOG PS, with the longest median treatment duration occurring in patients with a PS of 0 (4.5 months), followed by a PS of 1 (2.2 months), while patients with a PS of 2 + had a median treatment duration of 1.3 months and those of unknown PS had a median treatment duration of 3.3 months (). The relationship between the PS and line of pazopanib therapy is shown in Supplementary Table 3(C), with patients with PS 0/1 receiving pazopanib across a range of therapy lines.
Table 2A. Pazopanib treatment duration and clinical benefit by histological subtype.
Table 2B. Treatment duration by performance status (PS).
Effectiveness evaluations
Of the 211 patients, a clinical benefit at any time was experienced in 46% (97/211) of patients. Clinical benefit was observed in 62% (8/13) of first-line, 48% (28/59) of 2nd-line, 37% (22/60) of third-line, 58% (23/40) of fourth-line therapy and in 41% (16/39) of patients receiving pazopanib as a fifth-line therapy.
No CRs were observed. Patients with undifferentiated sarcoma (not otherwise specified (NOS)) had the highest percentage of PRs (26%, 5/19). Histological subtypes with the highest percentage of clinical benefit at any time, in sample sizes >10, included: nonuterine LMS (45%, 18/40) and uterine LMS (43%, 17/40), synovial sarcoma (54%, 13/24), undifferentiated sarcoma (NOS) (42%, 8/19), angiosarcoma (38%, 6/16) and solitary fibrous tumors (54%, 7/13) (). In groups with smaller sample sizes (5≥n> 10), 85% (4/5) of patients with desmoplastic small round cell tumors and 71% (5/7) of patients with LMS (of unknown location) experienced clinical benefit with pazopanib.
The median PFS was 3.0 months (95% CI: 2.7–3.5) ( and Supplementary Figure 3(A)). PFS decreased with a poorer PS, similar outcomes were observed for the PFS relating to each line therapy (). Median OS from first palliative treatment was 31.6 months (95% CI: 24.9–35.4) and from initiation of pazopanib therapy was 11.1 months (95% CI: 7.8–13.8) (, Supplementary Figure 3(B)).
Table 3. Progression-free survival (PFS) and overall survival (OS) for pazopanib therapy and survival status at end of study.
The OS from time of pazopanib initiation varied by histological subtypes, where the median OS (months) was similar for subjects with nonuterine LMS (12.9, CI: 6.1–17.3), synovial sarcoma (13.8, CI: 8.8–) and solitary fibrous tumor (13.2, CI: 4.5–25.7) and appears slightly longer for subjects with uterine LMS (16.5, CI: 5.5–24.7) and leiomyosarcoma of unknown location (17.6, CI: 4.7–NR) (Supplementary Table 4).
Adverse events
The most common AEs (>10%) with pazopanib (any grade) included: gastrointestinal disorders (i.e., diarrhea, nausea, vomiting, etc.) (28%, 60/211); general disorders (i.e., fatigue, asthenia, etc.) (27%, 56/211); skin and subcutaneous tissue disorders (18%, 38/211); and vascular disorders (i.e., hypertension, DVT and flushing) (11%, 24/211); however, none of these were of CTCAE version 4 grade 4 or 5 severity. shows the AEs of special interest. Thirteen percent (27/211) of patients discontinued due to AEs (Supplementary Table 5).
Table 4. Adverse events of special interest while on pazopanib therapy.
Discussion
The results of this retrospective chart review study demonstrate the activity of pazopanib in a mostly heavily pretreated aSTS patient population. The median duration of treatment of 3.1 months reflects the underlying condition of the patients receiving pazopanib therapy in this compassionate use setting, including patients that are quite often in poorer condition than in clinical studies, and receiving treatments that are not fully aligned with ESMO guidelines [Citation6] and therefore needs to be interpreted accordingly. Median treatment duration of pazopanib therapy varied considerably both by, and within, histological subtype.
Effectiveness was observed across a wide range of histological subtypes as demonstrated by extended duration of therapy in some patients, across most histological subtypes. While some patients having prolonged treatment were suffering from diseases with an indolent pattern of disease progression, for example, alveolar soft part sarcoma, the median duration of treatment in synovial sarcoma (5.1 months, n = 24) and desmoplastic small round cell tumor (5.7 months, n = 5), both aggressive, rapidly progressing diseases, is noteworthy. The small numbers make it difficult to draw firm conclusions about any specific subtype. It should also be noted that fibromatosis (n = 2) and sarcomatoid carcinoma (n = 1) are not strictly sarcomas, fibromatosis because it is a clonal connective tissue tumor that does not metastasize, but can be locally aggressive and occasionally fatal, and sarcomatoid carcinoma because it has epithelial differentiation but nevertheless may behave like a sarcoma and is often treated as one. However, these results are in accordance with previous publications in several case series of patients which have shown some patients with certain subtypes benefit from using pazopanib, including LMS, synovial sarcoma, angiosarcoma, UPS, MPNST, SFT, epithelioid hemangioendothelioma, hemangiopericytoma, rhabdomyosarcoma, desmoplastic small round cell and desmoid tumor/aggressive fibromatosis [Citation15–27]. Overall, in this study, 46% of patients experienced clinical benefit at any time while on pazopanib. However, it should be noted that response rate and PFS can be difficult to measure in the context of a retrospective study due to heterogeneity in scanning frequency and incomplete information in medical charts. The 1.3 month (range 1.1–2.3) treatment duration in ECOG PS 2+ patients echoes the limited role of chemotherapy in aSTS in these patients [Citation28]. Many patients (54%) had unknown PS in this study because this variable was not recorded in the medical charts. However, it is likely that this unknown PS was 0 or 1, aligned with the NPP recruitment requirements.
This NPP was not a controlled prospective clinical trial, and therefore, the experience with pazopanib in this study represents real-world effectiveness and safety of pazopanib used in a compassionate use setting in a heavily pretreated aSTS population. In addition, the absence of central radiological and pathological review due to the retrospective chart review methodology contributes uncertainty toward the outcomes reported here. The median OS (11.1 months) and PFS (3.0 months) observed supplement the results observed in the rigidly controlled phase-III PALETTE clinical trial study (OS of 12.5 months and PFS of 4.6 months), demonstrating that the activity with pazopanib translates into the compassionate use setting. It is important to remember that the effectiveness of the drug must be balanced with its cost [Citation29].
The extent and nature of the adverse events were consistent with the known safety profile of pazopanib with 13% of patients discontinuing due to adverse events [Citation5,Citation8,Citation10,Citation11,Citation13]. The adverse events that occurred were typically managed by temporary dose reductions of pazopanib. Although fewer AEs were reported in this study compared with PALETTE, this could be related to the retrospective nature of the study, as not all AEs may be fully captured in the charts. The tolerability of pazopanib was reassuring with a 92% relative dose intensity achieved, allowing most patients to receive close to the full 800 mg daily dose. However, this figure could have been influenced by failure to record all dose changes in the medical chart.
The results from the SPIRE study support the information generated from clinical trials showing pazopanib to be active in heavily pretreated patients and specifically after previous chemotherapy failure [Citation11,Citation13]. In this study, from a compassionate use program, 66% (139/211) of patients received pazopanib as a third or later line of therapy. Interestingly, many patients receiving treatment in the fourth line and fifth line and beyond setting still experienced a clinical benefit with pazopanib. In the present study, there was a distinct difference in treatment patterns for first-line compared to second and later lines of therapy. The dominant first-line treatment regimens were anthracyclines with or without ifosfamide, which is consistent with the recommendations in the guidelines for the treatment of aSTS, although this was also a prerequisite according to the eligibility criteria [Citation6]. In the second- and later-line settings, pazopanib was the dominant treatment regimen.
At time of chart abstraction for the SPIRE study, the majority of patients (60%, 127/211) received no further systemic treatment. Eighteen percent (15/84) of patients alive at the time of chart abstraction continued with pazopanib therapy.
Conclusions
The SPIRE study is one of the first datasets describing the activity of pazopanib in patients with aSTS outside of a clinical trial setting, building on the evidence from the small series of specific histological subtypes previously reported. Activity was evident across a wide range of histological subtypes which was encouraging in the context of this rare, heterogeneous group of diseases. No new safety concerns were noted and pazopanib was well tolerated with a dose intensity of 92%, equating to 715 mg daily dose. It is important to consider that this study was conducted in a patient population that received treatment exclusively in a compassionate use setting where patients typically have received multiple lines of treatment and have limited therapeutic options. Despite this, the data are reassuring and complement the existing clinical trial data, supporting the use of pazopanib in routine clinical practice.
Role of the funding source and contributors
Study investigators and GlaxoSmithKline were involved in writing the report and in the decision to submit for publication. GlaxoSmithKline employees (listed as authors) were involved in study design, data collection, interpretation, and analysis and writing of the report. This article was reviewed and approved by all authors. The corresponding author had full access to all the data in the study, was involved in report preparation and had final responsibility for the decision to submit for publication.
Financial support for medical editorial assistance was provided by GlaxoSmithKline. The authors thank J O’Regan (Bingham Mayne and Smith Ltd) for medical editorial assistance with the manuscript.
IONC_A_1332779_Supplementary_Information.zip
Download Zip (74.9 KB)Acknowledgments
This study was sponsored by GlaxoSmithKline; Pazopanib is property of Novartis Pharma AG as of 1 March 2015. We thank all institutions, patients, and their families, for their contributions to this study.
Disclosure statement
HG has no conflicts of interest. IJ, OM and PGC have received honoraria from GlaxoSmithKline; CB has received honoraria from GlaxoSmithKline, Pfizer, PharmaMar and Bayer; GG has received honoraria from GlaxoSmithKline, Pfizer, PharmaMar, Bayer and Novartis, DK received honoraria from Novartis, PGC has received honoraria from Amgen Dompé, ARIAD, Bayer, Blueprint Medicines, Eisai, Glaxo SK, Lilly, Merck SD, Merck Serono, Novartis, Pfizer, PharmaMar; KF is employed by UBC and DS was employed by UBC which received funding from GlaxoSmithKline for study conduct; MJ, AM and SCM were employees of and own shares in GlaxoSmithKline. SCM is currently an employee and owns shares in Novartis.
Additional information
Funding
References
- Burningham Z, Hashibe M, Spector L, et al. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2:14–29.
- Schöffski P, Cornillie J, Wozniak A, et al. Soft tissue sarcoma: an opdate on systemic treatment options for patients with advanced disease. Oncol Res Treat. 2014;37:355–362.
- Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109.
- Fletcher CDM, Bridge JA, Hogendoom PCW, et al. editors. WHO classification of tumors of soft tissue and bone, 4th ed. Lyon: IARC Press; 2013.
- Schöffski P. Pazopanib in the treatment of soft tissue sarcoma. Expert Rev Anticancer Ther. 2012;12:711–723.
- The ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii102–iii112.
- Leahy M, del Muro G, Reichardt P, et al. Chemotherapy treatment patterns and clinical outcomes in patients with metastatic soft tissue sarcoma. The Sarcoma treatment and Burden of Illness in North America and Europe (SABINE) study. Annals Oncol. 2012;23:2763–2770.
- Pazopanib. Summary of Product Characteristics; [cited 2016 Feb 10]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001141/WC500-094272.pdf.
- Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: clinical development of a potent anti-angiogenic drug. Crit Rev Oncol Hematol. 2011;77:163–171.
- Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer – Soft Tissue and Bone Sarcoma Group (EORTC Study 62043). J Clin Oncol. 2009;27:3126–3132.
- Van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886.
- Verweij J, Sleijfer S. Pazopanib, a new therapy for metastatic soft tissue sarcoma. Expert Opin Pharmacother. 2013;14:929–935.
- Gelderblom H, Benson C, Merimsky O, et al. Retrospective chart review of patients participating in the pazopanib sarcoma named patient programme - The SPIRE Study. 2014 Connective Tissue Oncology Society (CTOS) Annual Meeting, Berlin, Germany, 15–18 October 2014.
- Schur S, Lamm W, Koestler WJ, et al. Trabectedin in patients with metastatic soft tissue sarcoma: a retrospective single center analysis. Anticancer Drugs. 2013;24:725–730.
- Nakamura T, Matsumine A, Kawai A, et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: A Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer. 2016;122:1408–1416.
- Bally O, Tassy L, Richioud B, et al. Eight years tumor control with pazopanib for a metastatic resistant epithelioid hemangioendothelioma. Clin Sarcoma Res. 2015;5:12.
- Yoo KH, Kim HS, Lee SJ, et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer. 2015;15:154.
- Sedef AM, Köse F, Doğan Ö, et al. Targeted treatment with pazopanib in metastatic soft tissue sarcoma: Nearly complete response in two cases. Mol Clin Oncol 2015;3:400–402.
- Lee SJ, Kim ST, Park SH, et al. Successful use of pazopanib for treatment of refractory metastatic hemangiopericytoma. Clin Sarcoma Res. 2014;4:13.
- Stacchiotti S, Tortoreto M, Baldi GG, et al. Preclinical and clinical evidence of activity of pazopanib in solitary fibrous tumour. Eur J Cancer. 2014;50:3021–3028.
- Hashimoto A, Takada K, Takimoto R, et al. [Effective treatment of metastatic rhabdomyosarcoma with pazopanib]. Gan to Kagaku Ryoho. 2014;41:1041–1044.
- Frezza AM, Benson C, Judson IR, et al. Pazopanib in advanced desmoplastic small round cell tumours: a multi-institutional experience. Clin Sarcoma Res. 2014;4:7.
- Yamamoto Y, Nozawa M, Shimizu N, et al. Pazopanib for recurrent extraosseous Ewing's sarcoma of the retroperitoneum. Int J Urol. 2014;21:1183–1184.
- Martin-Liberal J, Benson C, McCarty H, et al. Pazopanib is an active treatment in desmoid tumour/aggressive fibromatosis. Clin Sarcoma Res. 2013;3:13.
- Campos SM, Brady WE, Moxley KM, et al. A phase II evaluation of pazopanib in the treatment of recurrent or persistent carcinosarcoma of the uterus: a gynecologic oncology group study. Gynecol Oncol. 2014;133:537–541.
- Tomita H, Koike Y, Asai M, et al. Angiosarcoma of the scalp successfully treated with pazopanib. J Am Acad Dermatol. 2014;70:e19–e21.
- Maruzzo M, Martin-Liberal J, Messiou C, et al. Pazopanib as first line treatment for solitary fibrous tumours: the Royal Marsden Hospital experience. Clin Sarcoma Res. 2015;5:5.
- Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens-a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17:150–157.
- Villa G, Hernández-Pastor LJ, Guix M, et al. Cost-effectiveness analysis of pazopanib in second-line treatment of advanced soft tissue sarcoma in Spain. Clin Transl Oncol. 2015;17:24–33.
