Abstract
Background: The aim of this meta-analysis was to systematically update the evidence for mindfulness-based stress reduction (MBSR) and mindfulness-based cognitive therapy (MBCT) in women with breast cancer.
Material and methods: In October 2016, PubMed, Scopus, and Central were searched for randomized controlled trials on MBSR/MBCT in breast cancer patients. The primary outcome was health-related quality of life. Secondary outcomes were fatigue, sleep stress, depression, anxiety, and safety. For each outcome, standardized mean differences (SMD/Hedges’ g) and 95% confidence intervals (CI) were calculated. Risk of bias was assessed by the Cochrane risk of bias tool.
Results: The Literature search identified 14 articles on 10 studies that included 1709 participants. The overall risk of bias was unclear, except for risk of low attrition bias and low other bias. Compared to usual care, significant post-intervention effects of MBSR/MBCT were found for health-related quality of life (SMD = .21; 95%CI = [.04–.39]), fatigue (SMD = −.28; 95%CI = [−.43 to −.14]), sleep (SMD = −.23; 95%CI = [−.40 to −.05]), stress (SMD = −.33; 95%CI = [−.61 to −.05]), anxiety (SMD = −.28; 95%CI = [−.39 to −.16]), and depression (SMD = −.34; 95%CI = [−.46 to −.21]). Up to 6 months after baseline effects were significant for: anxiety (SMD = −.28; 95%CI = [−.47 to −.09]) and depression (SMD = −.26; 95%CI = [−.47 to −.04]); and significant for anxiety (SMD = −.21; 95%CI = [−.40 to −.03]) up to 12 months after baseline. Compared to other active interventions, significant effects were only found post-intervention and only for anxiety (SMD = −.45; 95%CI = [−.71 to −.18]) and depression (SMD = −.39; 95%CI = [−.65 to −.14]). However, average effects were all below the threshold of minimal clinically important differences. Effects were robust against potential methodological bias. Adverse events were insufficiently reported.
Conclusions: This meta-analysis revealed evidence for the short-term effectiveness and safety of mindfulness-based interventions in women with breast cancer. However, their clinical relevance remains unclear. Further research is needed.
Background
Breast cancer has increased in prevalence since 1990–2015 and is the most common cancer diagnosis made worldwide [Citation1,Citation2]. Every year 1.7 million women are newly diagnosed, with most incidences reported from high-income countries [Citation3]. While the average number of years lived with disability has improved over the last decades [Citation2], patients’ health related quality of life (HQoL) including physical, psychological, and social continues to be affected by psychosocial symptoms and side effects of cancer treatment [Citation4]. In particular, cancer-related fatigue was found to significantly decrease HQoL [Citation5,Citation6], and sleep disturbances, stressful life events, and psychological distress contribute to even higher levels of breast-cancer mortality [Citation7–9].
In order to improve HQoL, symptoms and side effects, breast cancer patients frequently use complementary therapies [Citation10,Citation11]. A core construct of several complementary therapies is mindfulness [Citation12]. Derived from Buddhist tradition, the practice of mindfulness has been secularized and adapted for several patient groups [Citation13,Citation14]. Today, mindfulness focuses on self-regulation of attention and a state of consciousness that is associated with non-judgmental moment-to-moment awareness, patience and calmness, openness and trust, non-striving, letting go, and compassion [Citation15]. The most commonly used mindfulness-based interventions (MBI) are mindfulness-based stress reduction (MBSR) and mindfulness-based cognitive therapy (MBCT) [Citation15,Citation16]. MBSR is a structured 8-week group program of weekly sessions lasting an average of 2.5 h with an additional silent day retreat. Key components of MBSR comprise sitting and walking meditation, yogic exercises and mindful relaxation techniques. To incorporate mindfulness into everyday life, daily home practice is recommended and monitored [Citation14]. MBCT combines MBSR techniques with cognitive-behavioral methods such as psychoeducation, cognitive restructuring, and developing pleasant activities. It retains the 8-week group structure, the day retreat and daily home practice focusing on mindfulness exercises and psychoeducation [Citation17,Citation18].
In 2015, evidence for the effectiveness of MBSR and MBCT was reviewed in an overview of systematic reviews of randomized trials on MBSR or MBCT in different patient samples [Citation19]. This included five systematic reviews in mixed cancer populations and one in women with breast cancer from 2012 [Citation20] revealing significant small to medium effects on physical function, depression, anxiety, stress, and HQoL. In 2016, another meta-analysis of randomized controlled trials in breast cancer patients was published [Citation21]. But validity of the results is limited by: including only a patient subsample [Citation22] instead of the entire sample size [Citation23]; and by analyzing standard errors instead of standard deviations [Citation24]. In addition safety issues have not been investigated by previous reviews. As this was considered as essential for the estimation of the benefit-risk profile of an intervention [Citation25,Citation26], we aimed to systematically update and meta-analyze the available evidence for the effectiveness and safety of MBSR and MBCT in women diagnosed with breast cancer.
Material and methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) [Citation26] and the recommendations of the Cochrane Collaboration [Citation25].
Eligibility criteria
Types of studies: Randomized controlled trials (RCTs), cluster randomized controlled trials and randomized cross-over trials were eligible, if they were published as full-text articles in peer reviewed scientific journals.
Types of participants: Adults diagnosed with breast cancer (stage 0–IV) as defined by the American Joint Committee on Cancer tumor-node-metastasis (TNM) system [Citation27] regardless of current treatment status (undergoing or completed adjuvant treatment). Studies including heterogeneous cancer populations were excluded, except where outcomes for the breast cancer subgroup were reported separately.
Types of interventions: Groups that received MBSR or MBCT, or variations of these programs (regardless of program length, frequency, or duration of the treatment) were selected. Interventions that were clearly different to MBSR/MBCT, such as acceptance and commitment therapy [Citation28], mindfulness-based exercise [Citation29], or art therapy [Citation30] were excluded. Acceptable control interventions were usual care (no specific treatment/wait list) or any other active treatments.
Types of outcomes: Studies had to assess one primary outcome of patient self-reported health-related or cancer-related QoL post-intervention. Secondary outcomes included fatigue, sleep, stress, anxiety, depression, and safety. Intervention safety was assessed by the number of patients with adverse events. These are defined as any untoward medical occurrence in a patient administered intervention which does not necessarily have a causal relationship with this treatment [Citation31]. Serious adverse events are defined as cases of any untoward medical occurrence that, at any dose, has resulted in: death, was life-threatening, required inpatient hospitalization, or resulted in persistent or significant disability/incapacity [Citation31]. When more than one measure for an outcome was assessed in individual studies, standard instruments were preferred over novel ones, disease-specific over generic measures and multi-item over single-item measures. Time points for outcome assessment were designed as short-term, closest to 2 months after the start of the intervention (defined as post-intervention); medium-term, closest to 6 months after the start of the intervention and long-term, closest to 12 months after the start of the intervention.
Search strategy
In October 2016, electronic literature was systematically searched via PubMed (including Medline), Scopus (including Embase), and the Central without any restrictions for time or language (Supplementary Table S1). Additional manual searches included reference lists of identified original articles and published reviews, trial registries (who.int/ictrp and clinicaltrials.gov), and conference proceedings of scientific oncology and integrative medicine congresses [Citation32]. Identified abstracts were screened independently by two authors (HH and HC). Articles were read in full to assess eligibility. Disagreements were discussed with a third author (PK) until consensus was achieved.
Data extraction
Two authors (MW & PK) independently extracted data on characteristics of the: study population, intervention and control conditions, time points of data assessment after randomization, and outcomes included and not included in the meta-analysis. Discrepancies were rechecked with a third reviewer (HH) and discussed until consensus was achieved.
Risk of bias assessment
Risk of bias was assessed by two authors (MW & PK) independently using the Cochrane risk of bias tool [Citation25] and included the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. Each domain was judged as either, ‘low risk of bias’ if all requirements were adequately fulfilled, ‘high risk of bias’ if the requirements were not adequately fulfilled, and as ‘unclear risk of bias’ if insufficient data for a judgment was provided. Differing judgments were rechecked within the reviewer team (HH and HC) and discussed until consensus was achieved.
Statistical analyses
Data synthesis
Meta-analyses were conducted by Review Manager Software (RevMan, Version 5.3, The Nordic Cochrane Centre, Copenhagen) using random-effects models (inverse variance method) if at least two studies (k) assessed the respective continuous outcome in comparison to the respective control group at the respective time point. Effects were calculated as standardized mean differences (SMD) with 95% confidence intervals (CI) indicating the difference in means between groups divided by the pooled standard deviation (SD) using Hedges’ correction for small study samples (n) [Citation25]. For HQoL, positive SMDs indicated greater improvements due to mindfulness interventions. For fatigue, sleep disturbance, stress, anxiety, and depression, negative SMDs indicated greater reductions. If necessary, values were inverted. Effect sizes were categorized by Cohen’s classifications: SMD = .2–.5 = small effect, SMD = .5–.8 = medium effect, and SMD > .8 = large effect [Citation33].
To facilitate clinical interpretation, minimal clinically important differences (MCIDs) from selected questionnaires were compared to respective mean differences (MDs). MDs were calculated by multiplying SMDs and CIs by the pooled SD of baseline values [Citation25]. SDs were thereby retrieved from either epidemiological studies or RCTs using the respective instrument [Citation34–38].
Subgroup analyses
Subgroup analyses were intended for types of mindfulness intervention (MBSR vs. MBCT), cancer stage at randomization (non-metastatic vs. metastatic cancer), and treatment status (patients undergoing adjuvant treatment vs. those who had completed adjuvant treatment).
Dealing with missing data
Trial authors were contacted by email for missing means and SDs. In cases of no reply, SDs were calculated from standard errors (SE), CIs, or t-values [Citation25].
Assessment of heterogeneity
Statistical heterogeneity between studies was assessed by Chi2 statistics with a p-value of ≤ .10 indicating significant heterogeneity [Citation25]. The magnitude of heterogeneity was categorized by I2 statistics with I2 > 25%, I2 > 50%, and I2 > 75% representing moderate, substantial, and considerable heterogeneity, respectively [Citation25,Citation39].
Sensitivity analyses
To test the robustness of significant effects, sensitivity analyses were conducted for studies with high/unclear risk of bias versus low risk of bias at the domains: selection bias, performance bias, detection bias, and attrition bias. If substantial or considerable statistical heterogeneity was present in a respective meta-analysis, sensitivity analyses were also used to explore possible reasons for this.
Risk of bias across studies
We attempted to minimize risk of publication bias by searching international trial registries and conference proceedings for unpublished studies. Publication bias could not be assessed using funnel plots, as initially planned, because less than 10 studies were included in a single meta-analysis [Citation40].
Results
Search results
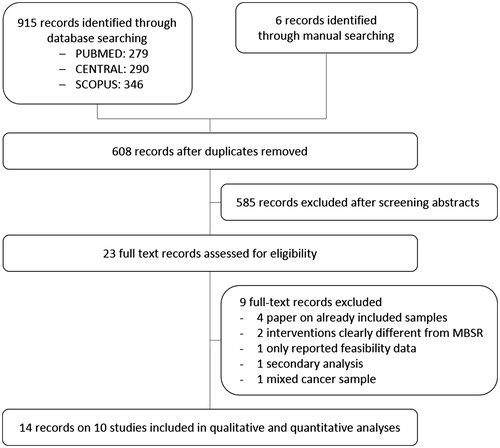
shows the electronic literature search flow chart of the 915 records identified. Manual searching revealed six additional unpublished studies awaiting classification. After removing duplicates and screening of abstracts, 23 full text records were assessed for eligibility. Out of these, nine further records were excluded because: samples were already included [Citation41–44], interventions clearly differed from MBSR/MBCT [Citation45,Citation46], only secondary analyses were performed [Citation47], only feasibility data were provided [Citation48], or only data of a mixed cancer sample were supplied [Citation49]. Missing data could be obtained from authors of eight records [Citation35,Citation50–56], while authors of one record confirmed that the published SDs are actually SEs [Citation24]. Finally, 14 records of 10 study samples with 1709 women could be included in the qualitative and quantitative analysis [Citation23,Citation24,Citation35,Citation37,Citation50–59].
Study characteristics
All included studies were RCTs comparing original MBSR programs [Citation50,Citation55–57], adapted MBSR programs [Citation23,Citation24,Citation35,Citation37,Citation51,Citation52,Citation54,Citation58,Citation59] or an adapted MBCT program [Citation53] to usual care [Citation50,Citation52,Citation59], enhanced usual care [Citation35,Citation51], wait list [Citation23,Citation24,Citation37,Citation53–55,Citation57–59], and/or other active comparators such as supportive expressive group therapy [Citation35,Citation51], or group nutrition education program [Citation52] (). Study samples were originated from the US [Citation23,Citation24,Citation37,Citation52,Citation54,Citation55,Citation58], Canada [Citation35,Citation51], the UK [Citation57], Denmark [Citation50,Citation53,Citation56], and China [Citation59] and mostly consisted of women with non-metastatic breast cancer [Citation23,Citation24,Citation37,Citation50,Citation52–59], except for one trial that did not plan to, but then included four women with TNM-stage IV metastatic breast cancer [Citation35,Citation51]. Cancer treatment status varied between studies with four RCTs including patients both under adjuvant and after adjuvant treatment [Citation50,Citation52,Citation55,Citation56,Citation59] and six RCTs only including patients who had completed adjuvant treatment [Citation23,Citation24,Citation35,Citation37,Citation51,Citation53,Citation54,Citation57,Citation58]. Further eligibility criteria were defined by one RCT [Citation35,Citation51], which included participants based on their baseline symptom level with regard to the primary outcome, while all other trials simply defined cancer-specific criteria. Sample sizes ranged from 44 to 336 with a median n of 146. Mean age ranged from 46.1 to 58.0 years with a median of 54.3 years.
Table 1. Characteristics of the included studies.
Risk of bias
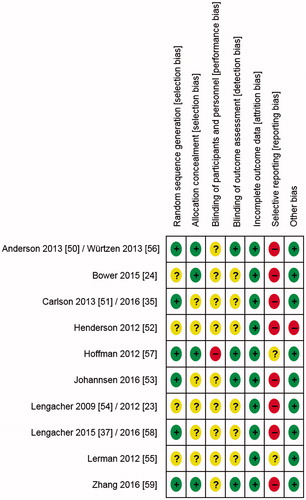
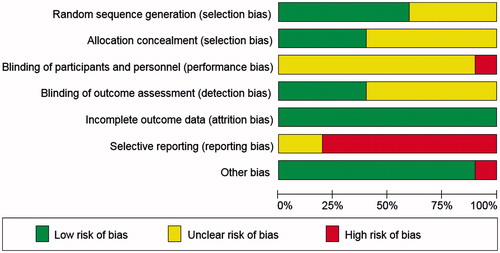
Risk of bias in included studies: and show the risk of bias judgments of these studies. Risk of selection bias was unclear in most trials; only three RCTs [Citation50,Citation56,Citation57,Citation59] reported adequate random sequence generation and allocation concealment. Risk of performance bias was consistently judged as unclear or high. Risk of detection bias was mainly unclear as well; only four RCTs reported adequate blinding of outcome assessors [Citation50,Citation53,Citation56,Citation57,Citation59]. Risk of attrition bias was low in all RCTs, by contrast risk of reporting bias was consistently unclear or high.
Risk of bias across studies: Although funnel plots could not be calculated, risk of publication bias, time lag bias, and language bias appeared to be low as all unpublished studies (NCT02840344, NCT01591915, NCT02125006, NCT02119481, NCT02647216, and DRKS00006015), retrieved from trial registries of 8 different countries, were still ongoing.
Overall comparisons
Primary outcome
HQoL
HQoL was assessed using two breast cancer specific measures, the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument (FACT-B) [Citation35,Citation51,Citation52,Citation57] and the European Organization of Research and Treatment of Cancer Breast specific module (EORTC QLQ-30 BR23) [Citation55], as well as generic tools including the Medical Outcomes Study Short Form Health Survey (SF-36) [Citation23,Citation37,Citation54,Citation58] and the World Health Organization Well-Being Index (WHO-5) [Citation53].
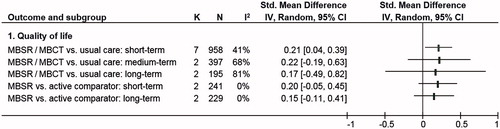
The meta-analysis on HQoL measures (; Supplementary Figure S1) revealed a statistically significant small short-term overall effect for MBSR/MBCT in comparison to usual care (k = 7; SMD = .21; 95%CI = [.04–.39]; p = .020; I2 = 41%; p = .120) [Citation51–55,Citation57,Citation58]. Compared to the MCID of the FACT-B, which was calculated as ≥7 points [Citation60], clinical relevance according to the converted MD of 4.39 (95%CI = [.84–8.15]) remains unclear as the CI included both clinically relevant and non-relevant group differences. Medium- and long-term comparisons were not statistically significant and showed substantial to considerable heterogeneity as well as contrary effects in the analyses of the respective k = 2 RCTs [Citation52,Citation53,Citation58] when compared to usual care. When compared to other active interventions, the meta-analysis on k = 2 RCTs [Citation35,Citation51,Citation52] homogeneously showed neither statistically significant short-term nor long-term effects in favor of MBSR. Medium-term effects could not be meta-analyzed because data were only available from k = 1 RCT [Citation35]. This RCT also showed no statistically significant effect of MBSR on HQoL in comparison to another active intervention.
Secondary outcomes
Fatigue
Fatigue was assessed by the Fatigue Symptom Inventory (FSI) [Citation24,Citation58], the fatigue severity subscale of the MD Anderson Symptom Inventory (MDASI) [Citation23], and the fatigue subscale of the Profile of Mood States (POMS) [Citation35,Citation51,Citation57].
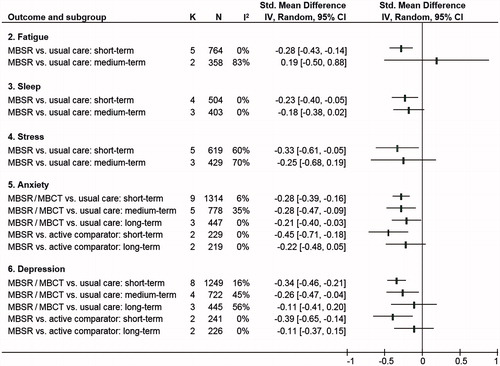
The meta-analysis on fatigue (; Supplementary Figure S2) revealed a statistically significant small short-term effect for MBSR compared to usual care (k = 5; SMD = −.28; 95%CI = [−.43–−.14]; p < .001; I2 = 0%; p = .510) [Citation23,Citation24,Citation51,Citation57,Citation58]. Compared to the MCID of the POMS, which was calculated as ≤5.6 points [Citation61], the corresponding MD of −2.04 (95%CI = [−3.14 to −1.02]) was not clinically relevant. The medium-term overall effect in comparison to usual care was not statistically significant. The analysis showed considerable heterogeneity and contrary effects between k = 2 RCTs [Citation24,Citation58]. Data for long-term comparisons of MBSR versus usual care were not available from the literature search. Pooled comparisons of MBSR against another active comparator could not be computed because data were only available from k = 1 RCT [Citation35], which showed statistically significant between-group differences in favor of MBSR at all three time points.
Sleep
Sleep disturbances were assessed using the following instruments: the Sleep Problem Index-II of the Medical Outcome Study Sleep Scale (MOS-SS) [Citation50], the Pittsburgh Sleep Quality Index (PSQI) [Citation24,Citation37], and the sleep impairment subscale of the MDASI [Citation23].
For short-term effects, meta-analysis of sleep measures revealed a statistically significant small group difference of MBSR in comparison to usual care (k = 4; SMD = −.23; 95%CI = [−.40 to −.05]; p = .001; I2 = 0%; p = .470) [Citation23,Citation24,Citation37,Citation50] (; Supplementary Figure S3). Compared to the MCID of the PSQI, which was calculated as ≤3 points [Citation62], the respective MD of −1.00 (95%CI = [−1.74 to −.22]) was not clinically relevant. Pooled medium-term effects of k = 3 RCTs [Citation24,Citation37,Citation50] homogeneously were very small and did not statistically significantly favor MBSR. Effects at long-term could not be meta-analyzed because only k = 1 RCT [Citation50] assessed long-term data in comparison to usual care. That study also reported no statistically significant effects on sleep disturbances in comparisons to usual care. Effects of MBSR against other active interventions could not be meta-analyzed because data were not available.
Stress
Stress questionnaires included the Perceived Stress Scale (PSS) [Citation24,Citation54,Citation58,Citation59] and the Calgary Symptoms of Stress Inventory (C-SOSI) [Citation35,Citation51].
In comparison to usual care, meta-analysis showed a statistically significant small effect of MBSR on stress (k = 5; SMD = −.33; 95%CI = [−.61 to −.05]; p = .020; I2 = 60%; p = .040) [Citation24,Citation51,Citation54,Citation58,Citation59] with, however, substantial heterogeneity between the included RCTs. Compared to the MCID of the PSS, which was calculated as ≤11 points [Citation63], the respective MD of −2.49 (95%CI = [−4.61 to −.38]) was not clinically relevant. Medium-term effects of k = 3 RCTs [Citation24,Citation58,Citation59] that compared MBSR/MBCT to usual care also showed substantial heterogeneity but no statistically significant pooled effect (; Supplementary Figure S4). None of the RCTs with usual care as a control group assessed long-term effects. Effects of MBSR against other active interventions could not be meta-analyzed as they were reported by only k = 1 RCT [Citation35], which found small statistically significant short-term but no long-term effects.
Anxiety
Studies assessing anxiety used the following instruments: the anxiety subscale of the SCL-90-R [Citation56], the fear of recurrence subscale of the QoL in Adult Cancer Survivors (QLACS) [Citation24], the anxiety subscale of the POMS [Citation35,Citation51,Citation57], the anxiety subscale of the Courtauld Emotional Control Scale (CEC) [Citation52], the Hospital Anxiety Scale (HADS-A) [Citation53], and the state anxiety subscale of the State Trait Anxiety Inventory (STAI-S) [Citation54,Citation58,Citation59]. Anxiety data from 5 publications were not included in the meta-analysis because: (i) a more comprehensive anxiety measure used in the respective study was already included [Citation58], (ii) state anxiety data were preferred over trait data [Citation54,Citation59], (iii) published were preferred over unpublished data [Citation52], and (iv) anxiety data were only available for the mixed cancer sample but not for the subsample of women with breast cancer [Citation55].
Short-, medium-, and long-term effects of MBSR/MBCT in comparison to usual care were found to be homogeneously small and statistically significant: short-term (k = 9; SMD = −.28; 95%CI = [−.39 to −.16]; p < .001; I2 = 6%; p = .380) [Citation24,Citation51–54,Citation56–59], medium-term (k = 5; SMD = −.28; 95%CI = [−.47 to −.09]; p = .004; I2 = 35%; p = .190) [Citation24,Citation53,Citation56,Citation58,Citation59], and long-term effects (k = 3; SMD = −.21; 95%CI = [−.40 to −.03]; p = .002; I2 = 0%; p = .930) [Citation52,Citation53,Citation56]. However, the converted short-term MD of −1.03 (95%CI = [−1.44 to −.59]), the medium-term MD of −1.03 (95%CI = [−1.73 to −.33]) as well as the long-term MD of −.08 (95%CI = [−1.47 to −.11]) compared to the MCID of the HADS-A, calculated as ≤2 points [Citation64], were not clinically relevant. Comparisons to other active interventions also revealed a statistically significant small effect in favor of MBSR for anxiety, but only for short-term (k = 2; SMD = −.45; 95%CI = [−.71 to −.18]; p < .001; I2 = 0%; p = .460) [Citation51,Citation52] and only in the range of questionable clinical relevance according to a MD of −1.66 (95%CI = [−2.61 to −.66]). Medium-term comparisons could not be computed due to insufficient published data; pooled long-term effects of k = 2 RCTs [Citation35,Citation52] on MBSR versus other active interventions were found to be not statistically significant (; Supplementary Figure S5).
Depression
Depression was assessed using the following questionnaires: the Center for Epidemiological Studies Depression Scale (CES-D) [Citation24,Citation54,Citation56,Citation58], the anxiety subscale of the POMS [Citation35,Citation51,Citation57], the depression subscale of the SCL-90-R [Citation52], and the Hospital Depression Scale (HADS-D) [Citation53]. Depression data, assessed by two studies, were not included in the meta-analysis as published data were preferred over unpublished data [Citation52,Citation56].
In comparison to usual care, meta-analysis revealed statistically significant small group differences in favor of MBSR/MBCT for short-term (k = 8; SMD = −.34; 95%CI = [−.46 to −.21]; p < .001; I2 = 16%; p = .300) [Citation24,Citation51–54,Citation56–58] and for medium-term comparisons (k = 4; SMD = −.26; 95%CI = [−.47 to −.04]; p = .020; I2 = 45%; p = .140) [Citation24,Citation53,Citation56,Citation58]. However, the corresponding short-term MD of −1.17 (95%CI = [−1.59 to −.72]) and the medium-term MD of −.90 (95%CI = [−1.62 to −.14]) compared to the MCID of the HADS-D, calculated as ≤1.9 points [Citation65], were not clinically relevant. The analyses, k = 3 RCTs [Citation52,Citation53,Citation56], of the long-term effects of MBSR/MBCT on depression, in comparison to usual care, were not statistically significant. When MBSR was compared to other active interventions, a statistically significant small effect for depression was found for short-term (k = 2; SMD = −.39; 95%CI = [−.65 to −.14]; p = .002; I2 = 0%; p = .960) [Citation51,Citation52], but not for long-term effects [Citation35,Citation52] (Supplementary Figure S6). With a short-term MD of 1.15 (95%CI = [−2.24 to −.48]) clinical relevance remains unclear as the CI included both clinically relevant and non-relevant group differences. Medium-term analysis could not be computed because of insufficient published data.
Safety
Safety issues were reported by only k = 2 of the included studies, which stated that no serious adverse events occurred [Citation56,Citation57]. All other trials report neither occurrence nor absence of adverse events. However, reasons for discontinuing the MBSR/MBCT intervention or drop-out from the follow-up assessment were reported by all k = 10 studies. Besides scheduling and motivational problems, reasons included breast cancer recurrence (n = 3) [Citation54,Citation57], illness other than breast cancer (n = 9) [Citation56,Citation57], and unknown reasons (n = 135) [Citation24,Citation35,Citation51–53,Citation55–59].
Subgroup analyses
Type of intervention
Subgroup analyses of MBSR in contrast to MBCT could not be computed separately because only k = 1 RCT [Citation53] investigated MBCT in comparison to usual care. In this RCT, statistically significant short-term effects were reported for HQoL and anxiety; medium-term effects for HQoL and depression; long-term effects only for HQoL.
By excluding the MBCT data from meta-analyses, statistically significant short-term effects of MBSR in comparison to usual care could be detected for anxiety (k = 8; SMD = .27; 95%CI = [−.39 to −.14]; p < .001; I2 = 13%; p = .330) [Citation24,Citation51,Citation52,Citation54,Citation56–59] and depression (k = 7; SMD = −.34; 95%CI = [−.48 to −.19]; p < .001; I2 = 27%; p = .220) [Citation24,Citation51,Citation52,Citation54,Citation56–58], while HQoL was no longer statistically significant (k = 6; SMD = .15; 95%CI = [−.00 to .30]; p = .06; I2 = 14%; p = .330) [Citation51,Citation52,Citation54,Citation55,Citation57,Citation58]. Further comparisons to usual care on fatigue, sleep, and stress, which revealed statistically significant short-term effects, did not contain MBCT data. Statistically significant medium-term effects of MBSR in comparison to usual care were found for anxiety (k = 4; SMD = −.27; 95%CI = [−.50 to −.04]; p = .020; I2 = 46%; p = .130) [Citation24,Citation56,Citation58,Citation59]. But medium-term effects for depression, based only on MBSR trials, were no longer statistically significant (k = 3; SMD = −.18; 95%CI = [−.36 to .01]; p = .060; I2 = 18%; p = .290) [Citation24,Citation56,Citation58]. Excluding MBCT from the analyses led to no statistically significant long-term effects of MBSR in comparison to usual care, neither for anxiety (k = 2; SMD = −.21; 95%CI = [−.42 to .00]; p = .050; I2 = 0%; p = .730) [Citation52,Citation56]. Comparisons to active comparators were only based on MBSR data.
Cancer stage
Subgroup analyses of trials examining non-metastatic versus metastatic cancer patients revealed only k = 1 RCT [Citation35,Citation51] that also included women with TNM-stage IV metastatic breast cancer. By excluding this RCT from the respective meta-analyses, results did not change.
Treatment status
For samples that only included women under adjuvant treatment, statistically significant short-term effects of MBSR in comparison to usual care were found for: sleep (k = 1) [Citation50], stress (k = 1) [Citation59], anxiety (k = 3; SMD = −.30; 95%CI = [−.57 to .04]; p = .020; I2 = 35%; p = .210) [Citation52,Citation56,Citation59], and depression (k = 2; SMD = −.34; 95%CI = [−.54 to .13]; p = .001; I2 = 0%; p = .510) [Citation52,Citation56], while HQoL no longer showed statistically significant results (k = 2; SMD = .22; 95%CI = [−.28 to .71]; p = .400; I2 = 45%; p = .180) [Citation52,Citation55]. Fatigue was not investigated as an outcome for MBSR/MBCT in comparison to usual care for women undergoing adjuvant treatment. Statistically significant medium-term effects in comparison to usual care were found for: stress (k = 1) [Citation59] and depression (k = 1) [Citation56], while sleep (k = 1) [Citation50] and anxiety (k = 2; SMD = −.46; 95%CI = [−.57 to .05]; p = .080; I2 = 68%; p = .080) [Citation56,Citation59] were not found to be statistically significant. Medium-effects for HQoL were not assessed in individual studies on women under adjuvant treatment. Comparisons of long-term effects to usual care did not include sleep and stress nor were they statistically significant for HQoL (k = 1) [Citation52], anxiety (k = 2; SMD = −.21; 95%CI = [−.42 to .00]; p = .050; I2 = 0%; p = .730) [Citation52,Citation56], and depression (k = 2; SMD = −.03; 95%CI = [−.51 to .45]; p = .910; I2 = 76%; p = .040) [Citation52,Citation56]. When MBSR were compared to active interventions, statistically significant effects were only found for short-term anxiety and depression, all based on k = 1 RCT [Citation52], while effects on HQoL were not found be statistically significant for short- and long-term effects [Citation52]. For sleep, fatigue, and stress, no data were available.
In studies examining woman who completed adjuvant treatment, short-term comparisons of MBSR/MBCT to usual care were found to be statistically significant for HQoL (k = 5; SMD = .23; 95%CI = [.01–.44]; p = .040; I2 = 51%; p = .080) [Citation51,Citation53,Citation54,Citation57,Citation58], fatigue (k = 5; SMD = −.28; 95%CI = [−.43 to −.14]; p < .001; I2 = 0%; p = .510) [Citation23,Citation24,Citation51,Citation57,Citation58], anxiety (k = 6; SMD = −.28; 95%CI = [−.42 to −.14]; p < .001; I2 = 8%; p = .360) [Citation24,Citation51,Citation53,Citation54,Citation57,Citation58], and depression (k = 6; SMD = −.36; 95%CI = [−.53 to −.18]; p < .001; I2 = 37%; p = .160) [Citation24,Citation51,Citation53,Citation54,Citation57,Citation58], but not for sleep (k = 3; SMD = −.23; 95%CI = [−.53 to .07]; p = .130; I2 = 22%; p = .280) [Citation23,Citation24,Citation37] nor for stress (k = 4; SMD = −.27; 95%CI = [−.56 to .02]; p = .070; I2 = 58%; p = .070) [Citation24,Citation51,Citation54,Citation58]. None of the outcomes were statistically significant for medium-term comparisons against usual care as for HQoL and fatigue analyses did not differ from overall comparisons; and for sleep (k = 2; SMD = −.14; 95%CI = [−.48 to .19]; p = .400; I2 = 0%; p = .960) [Citation24,Citation37], stress (k = 2; SMD = −.02; 95%CI = [−.22 to .18]; p = .850; I2 = 0%; p = .650) [Citation24,Citation58], anxiety (k = 3; SMD = −.17; 95%CI = [−.36 to .01]; p = .060; I2 = 0%; p = .430) [Citation24,Citation53,Citation58], and depression (k = 3; SMD = −.29; 95%CI = [−.63 to .06]; p = .100; I2 = 62%; p = .070) [Citation24,Citation53,Citation58], comparisons were not statistically significant. Long-term effects against usual care were only assessed for HQoL, anxiety and depression and only by k = 1 trial [Citation53] showing no statistically significant differences between groups. When MBSR was compared to other active interventions, statistically significant short-term effects, based on k = 1 RCT [Citation35], could be found for fatigue, anxiety, and depression; and statistically significant medium-term results for fatigue.
Sensitivity analysis
By excluding all studies with high and unclear risk of selection bias, all short-term effects of MBSR/MBCT in comparison to usual care remained statistically significant, except for sleep. Previous statistically significant medium-term-effects found for anxiety and depression remained so only for depression; long-term effects on anxiety were no longer found to be statistically significant. When MBSR/MBCT was compared to other active interventions, meta-analysis of short-term effects on depression, which had revealed significant differences, could no longer be computed because there were no studies with low risk of selection bias. Observed results for sleep, anxiety, and depression were now based on only one trial with CIs very close to zero, which could only be interpreted with restraint. Further sensitivity analyses revealed the same results for studies with low versus unclear detection bias. Studies with low performance bias were not available, while all studies included in the meta-analysis were judged as low risk for attrition bias.
Discussion
This systematic review found evidence for statistically significant short-term effects of MBSR and MBCT in comparison to usual care on HQoL, fatigue, sleep, stress, anxiety, and depression. However, effect sizes were small [Citation33] and average effects did not reach MCID thresholds.
Up to 6 months, small effects remained statistically significant for anxiety and depression. Up to 12 months, statistically significant effects were found only for anxiety. For both time points and outcomes, effect sizes were again small [Citation33] and average effects did not reach MCID thresholds. While short-term results were mostly homogeneous, except for stress, medium- and long-term comparisons of MBSR/MBCT versus usual care were often based on substantial statistical heterogeneity as well as a low number of included RCTs. They should, therefore, be interpreted with restraint until further trials confirm or refute the results.
In comparison to other active interventions, meta-analysis homogeneously revealed statistically significant short-term effects of MBSR and MBCT for anxiety and depression. These were categorized as small [Citation33] and below the threshold of the respective MCIDs. Medium-term data for 6-months analyses of MBSR or MBCT in comparison to active interventions were not available from the literature search; data for 12-months analyses no longer showed statistically significant pooled effects. However the results should be viewed in the light of there being no more than 2 available RCTs for the respective meta-analyses.
In subgroup analyses, MBSR and MBCT were found to be equally effective for women under adjuvant treatment and survivors. For comparisons of different intervention types and TNM-stages, evidence was currently insufficient to draw any conclusions.
In comparison to the 2012 review on MBSR for breast cancer [Citation20], the literature search identified eight further RCTs resulting in more precise, smaller estimates of effect sizes and CIs. Former meta-analyses that included RCTs and non-RCTs on MBSR for breast cancer [Citation65,Citation66] had shown greater effect sizes. The aforementioned 2015 overview of systematic reviews of RCTs in different patient samples [Citation19] revealed equal effect sizes for HQoL and depression as compared to wait list/usual care, while effects on stress and anxiety appear to be greater in RCTs of healthy people and other chronic physical and mental conditions.
The limitations of the present study include (i) the overall unclear risk of bias. However, results did not change considerably when only studies with low risk of selection bias, detection bias or attrition bias were included in the meta-analysis. In addition, as there was a small number of included studies, risk of publication bias could not be ruled out. (ii) Safety issues, moreover, could not be assessed adequately as only three trials reported adverse events or reasons for drop-out. Those could be classified as not serious except in two cases of breast cancer recurrence, where MBSR/MBCT cannot be excluded as a cause but would appear unlikely. (iii) Evidence might be biased by the small number of RCTs used in several of the meta-analyses. Conclusions drawn, especially those from k = 2 analyzes, remain preliminary. (iv) Moreover, the inclusion of non-peer reviewed data, retrieved by the trial authors upon request, might have introduced bias. (v) Heterogeneity might be an additional source for bias. Neither clinical heterogeneity (no decrease in statistical heterogeneity in subgroup analyses) nor methodological heterogeneity (no decrease in statistical heterogeneity in sensitivity analyses) could explain the statistical heterogeneity of effects.
Further clinical trials on MBSR and MBCT for women with breast cancer are required. This meta-analysis could not draw any conclusions for women with metastatic breast cancer nor for longer term follow-ups. As outcome domains varied a lot between studies, future trials should select standardized outcome clusters with high relevance for women with breast cancer as for example recommended by Reich et al. [Citation67]. Moreover, future trials should ensure rigorous methodology and reporting and should focus on developing adequate control groups that better ensure controlling for unspecific therapy and therapist effects in order to reduce risk of performance bias. Finally, increased attention should be paid to adequately assessing and reporting adverse events and reasons for drop-out.
Implications for clinical practice should be drawn in the light of these limitations. This meta-analysis suggests short-term effectiveness and safety of MBSR/MBCT interventions for women diagnosed with breast cancer during and subsequent to adjuvant treatment. Therefore, offering MBSR/MBCT courses to such patients could might provide an additional option in supportive cancer care.
Conclusions
This systematic review found preliminary evidence of the safety and short-term effectiveness of MBIs in women with breast cancer for HQoL, fatigue, sleep, stress, anxiety, and depression. However, the clinical relevance remains unclear from most of the findings. Further trials with longer follow-up periods and more active control conditions are needed before MBIs can definitely be recommended for women with breast cancer.
Other publications
An abstract of this manuscript was accepted for presentation at the World Congress on Integrative Medicine & Health, May 2017, Berlin, Germany. An abstract was published in BMC Complementary and Alternative Medicine.
Supplementary_figures.zip
Download Zip (949.7 KB)Disclosure statement
All authors disclose any commercial association that might create a conflict of interest regarding the submitted manuscript. There is especially no competing financial interest for any of the authors.
References
- Global Burden of Disease Study Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800.
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
- Brandao T, Schulz MS, Matos PM. Psychological adjustment after breast cancer: a systematic review of longitudinal studies. Psychooncology 2016; doi: 10.1002/pon.4230. [Epub ahead of print].
- Alcantara-Silva TR, Freitas-Junior R, Freitas NM, et al. Fatigue related to radiotherapy for breast and/or gynaecological cancer: a systematic review. J Clin Nurs. 2013;22:2679–2686.
- Abrahams HJ, Gielissen MF, Schmits IC, et al. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. 2016;27:965–974.
- Chida Y, Hamer M, Wardle J, et al. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Prac Oncol. 2008;5:466–475.
- Lin X, Chen W, Wei F, et al. Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med. 2015;16:1381–1387.
- Suppli NP, Johansen C, Kessing LV, et al. Survival after early-stage breast cancer of women previously treated for depression: a nationwide Danish cohort study. J Clin Oncol. 2017;35:334–342.
- Fouladbakhsh JM, Stommel M. Gender, symptom experience, and use of complementary and alternative medicine practices among cancer survivors in the U.S. cancer population. Oncol Nurs Forum. 2010;37:E7–E15.
- Neuhouser ML, Smith AW, George SM, et al. Use of complementary and alternative medicine and breast cancer survival in the Health, Eating, Activity, and Lifestyle Study. Breast Cancer Res Treat. 2016;160:539–546.
- Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA 2008;300:1350–1352.
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4:33–47.
- Kabat-Zinn J, Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. New York (NY): Delta Trade Paperback/Bantam Dell; 1990.
- Bishop SR, Lau M, Shapiro S, et al. Mindfulness: a proposed operational definition. Clin Psychol Sci Pract. 2004;11:230–241.
- Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Pract. 2003;10:125–143.
- Teasdale JD, Segal ZV, Williams JM, et al. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68:615–623.
- Crane R. Mindfulness-based cognitive therapy: distinctive features. New York (NY): Routledge/Taylor & Francis Group; 2009.
- Gotink RA, Chu P, Busschbach JJ, et al. Standardised mindfulness-based interventions in healthcare: an overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10:e0124344.
- Cramer H, Lauche R, Paul A, et al. Mindfulness-based stress reduction for breast cancer-a systematic review and meta-analysis. Curr Oncol. 2012;19:e343–e352.
- Zhang J, Xu R, Wang B, et al. Effects of mindfulness-based therapy for patients with breast cancer: a systematic review and meta-analysis. Complement Ther Med. 2016;26:1–10.
- Reich RR, Lengacher CA, Kip KE, et al. Baseline immune biomarkers as predictors of MBSR(BC) treatment success in off-treatment breast cancer patients. Biol Res Nurs. 2014;16:429–437.
- Lengacher CA, Reich RR, Post-White J, et al. Mindfulness based stress reduction in post-treatment breast cancer patients: an examination of symptoms and symptom clusters. J Behav Med. 2012;35:86–94.
- Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121:1231–1240.
- Higgins JPT, Green S. Cochrane Handbook for systematic reviews of interventions Version 5.1.0: The Cochrane Collaboration; [Internet]. 2011. Available from: http://handbook.cochrane.org.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341.
- Compton CC, Byrd DR, Garcia-Aguilar J, et al. AJCC Cancer Staging Atlas. A Companion to the Seventh Editions of the AJCC Cancer Staging Manual and Handbook. New York: Springer; 2012.
- Graham CD, Gouick J, Krahe C, et al. A systematic review of the use of Acceptance and Commitment Therapy (ACT) in chronic disease and long-term conditions. Clin Psychol Rev. 2016;46:46–58.
- Tacon AM, McComb J. Mindful exercise, quality of life, and survival: a mindfulness-based exercise program for women with breast cancer. J Altern Complement Med. 2009;15:41–46.
- Jang SH, Kang SY, Lee HJ, et al. Beneficial effect of mindfulness-based art therapy in patients with breast cancer – a randomized controlled trial. Explore (NY). 2016;12:333–340.
- European Medicines Agency. ICH Harmonized Tripartite Guideline E6: Note for Guidance on Good Clinical Practice (PMP/ICH/135/95). London: European Medicines Agency; 2002.
- Cramer H, Lauche R, Klose P, et al. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017;1:CD010802.
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum Associates; 1988.
- Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986.
- Carlson LE, Tamagawa R, Stephen J, et al. Randomized-controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy among distressed breast cancer survivors (MINDSET). Psychooncology. 2016;25:750–759.
- Cohen S, Janicki-Deverts D. Who's stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 20091. J Appl Soc Psychol. 2012;42:1320–1334.
- Lengacher CA, Reich RR, Paterson CL, et al. The effects of mindfulness-based stress reduction on objective and subjective sleep parameters in women with breast cancer: a randomized controlled trial. Psychooncology. 2015;24:424–432.
- Ng CG, Mohamed S, Kaur K, et al. Perceived distress and its association with depression and anxiety in breast cancer patients. PLoS One. 2017;12:e0172975.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560.
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634.
- Carlson LE, Beattie TL, Giese-Davis J, et al. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer. 2015;121:476–484.
- Hebert JR, Ebbeling CB, Olendzki BC, et al. Change in women's diet and body mass following intensive intervention for early-stage breast cancer. J Am Diet Assoc. 2001;101:421–431.
- Henderson VP, Massion AO, Clemow L, et al. A randomized controlled trial of mindfulness-based stress reduction for women with early-stage breast cancer receiving radiotherapy. Integr Cancer Ther. 2013;12:404–413.
- Wurtzen H, Dalton SO, Christensen J, et al. Effect of mindfulness-based stress reduction on somatic symptoms, distress, mindfulness and spiritual wellbeing in women with breast cancer: results of a randomized controlled trial. Acta Oncol. 2015;54:712–719.
- Spahn G, Choi KE, Kennemann C, et al. Can a multimodal mind-body program enhance the treatment effects of physical activity in breast cancer survivors with chronic tumor-associated fatigue? A randomized controlled trial. Integr Cancer Ther. 2013;12:291–300.
- Targ EF, Levine EG. The efficacy of a mind-body-spirit group for women with breast cancer: a randomized controlled trial. Gen Hosp Psychiatry. 2002;24:238–248.
- Shapiro SL, Bootzin RR, Figueredo AJ, et al. The efficacy of mindfulness-based stress reduction in the treatment of sleep disturbance in women with breast cancer: an exploratory study. J Psychosom Res. 2003;54:85–91.
- Leydon GM, Eyles C, Lewith GT. A mixed methods feasibility study of mindfulness meditation for fatigue in women with metastatic breast cancer. Eur J Integr Med. 2012;4:e429–e435.
- van der Lee ML, Garssen B. Mindfulness-based cognitive therapy reduces chronic cancer-related fatigue: a treatment study. Psychooncology 2012;21:264–272.
- Andersen SR, Wurtzen H, Steding-Jessen M, et al. Effect of mindfulness-based stress reduction on sleep quality: results of a randomized trial among Danish breast cancer patients. Acta Oncol. 2013;52:336–344.
- Carlson LE, Doll R, Stephen J, et al. Randomized controlled trial of Mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer. J Clin Oncol. 2013;31:3119–3126.
- Henderson VP, Clemow L, Massion AO, et al. The effects of mindfulness-based stress reduction on psychosocial outcomes and quality of life in early-stage breast cancer patients: a randomized trial. Breast Cancer Res Treat. 2012;131:99–109.
- Johannsen M, O'connor M, O'toole MS, et al. Efficacy of mindfulness-based cognitive therapy on late post-treatment pain in women treated for primary breast cancer: a randomized controlled trial. J Clin Oncol. 2016;34:3390–3399.
- Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology 2009;18:1261–1272.
- Lerman R, Jarski R, Rea H, et al. Improving symptoms and quality of life of female cancer survivors: a randomized controlled study. Ann Surg Oncol. 2012;19:373–378.
- Würtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49:1365–1373.
- Hoffman CJ, Ersser SJ, Hopkinson JB, et al. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30:1335–1342.
- Lengacher CA, Reich RR, Paterson CL, et al. Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2016;34:2827–2834.
- Zhang JY, Zhou YQ, Feng ZW, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) on posttraumatic growth of Chinese breast cancer survivors. Psychol Health Med. 2016;22:94–109.
- Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57:898–910.
- Nordin A, Taft C, Lundgren-Nilsson A, et al. Minimal important differences for fatigue patient reported outcome measures-a systematic review. BMC Med Res Methodol. 2016;16:62.
- Backhaus J, Junghanns K, Broocks A, et al. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–740.
- Eskildsen A, Dalgaard VL, Nielsen KJ, et al. Cross-cultural adaptation and validation of the Danish consensus version of the 10-item Perceived Stress Scale. Scand J Work Environ Health. 2015;41:486–490.
- Chan KS, Aronson Friedman L, Bienvenu OJ, et al. Distribution-based estimates of minimal important difference for hospital anxiety and depression scale and impact of event scale-revised in survivors of acute respiratory failure. Gen Hosp Psychiatry. 2016;42:32–35.
- Zainal NZ, Booth S, Huppert FA. The efficacy of mindfulness-based stress reduction on mental health of breast cancer patients: a meta-analysis. Psychooncology. 2013;22:1457–1465.
- Huang HP, He M, Wang HY, et al. A meta-analysis of the benefits of mindfulness-based stress reduction (MBSR) on psychological function among breast cancer (BC) survivors. Breast Cancer. 2016;23:568–576.
- Reich RR, Lengacher CA, Alinat CB, et al. Mindfulness-based stress reduction in post-treatment breast cancer patients: immediate and sustained effects across multiple symptom clusters. J Pain Symptom Manage. 2017;53:85–95.