Abstract
Background: 68Ga-DOTANOC PET/CT is routinely used to image neuroendocrine tumors (NETs). A case of lymphoma initially thought to be NET based on a positive 68Ga-DOTANOC PET/CT was recently seen at our institution. This prompted us to determine prospectively somatostatin receptor (SSTR) status in patients with lymphoma by immunohistochemical analysis of SSTR subtypes 2, 3 and 5 (SSTR2,3,5) and 68Ga-DOTANOC PET/CT imaging.
Material and methods: Twenty-one patients with newly diagnosed lymphoma were referred to 68Ga-DOTANOC and FDG PET/CT prior to any treatment. Tracer uptake was evaluated visually by two nuclear medicine specialists. Maximum standardized uptake values (SUVmax) were determined from 14 nodal and two extranodal regions with highest uptake in each patient. Lesions were then graded with Deauville score (1–5) on FDG PET/CT and modified Krenning score (0–4) on 68Ga-DOTANOC PET/CT, respectively. SSTR2,3,5 status was analyzed from routine biopsies of lymphomatous tissue and matched to corresponding PET/CT findings.
Results: About 20/21 patients had FDG-positive lymphoma (Deauville score ≥3). Uptake of 68Ga-DOTANOC was regarded as positive if Krenning score was ≥2 and resulted in 13/21 (62%) patients having 68Ga-DOTANOC-positive lymphomas. The highest uptake of 68Ga-DOTANOC was seen in Hodgkin’s lymphoma of nodular sclerosis subtype and in diffuse large B-cell lymphoma (SUVmax median 9.8 and 9.7, respectively). Both cases showed strong SSTR2 immunopositivity in tumor cells. Some patients had SSTR2 immunopositivity predominantly in endothelial and dendritic cells and follicular centers of lymph nodes contributing to a positive PET/CT with probably low tumor-specific uptake. SSTR3 and SSTR5 were negative in most lymphoma subtypes.
Conclusions: According to this pilot study, 68Ga-DOTANOC PET/CT is positive in some lymphoma subtypes which express SSTRs. These tumors present a potential risk of being misinterpreted as NETs if a representative tumor sample is not available. Lymphomas with high expression of SSTRs may be amenable to treatments targeting these receptors.
Background
Somatostatin receptor (SSTR) imaging with 68Ga-DOTA-conjugated peptides can be exploited both for diagnostic and theranostic purposes at PET/CT using a variety of octreotide analogs. For instance, 68Ga-DOTA-1-Nal3-octreotide (68Ga-DOTANOC) binds to SSTR subtypes 2, 3 and 5 (SSTR2,3,5) with high affinity, while 68Ga-DOTA0-Tyr3-octreotate (68Ga-DOTATATE) binds more selectively only to SSTR2 [Citation1,Citation2]. Clearly, radiolabelled octreotide imaging is the method of choice to detect differentiated neuroendocrine tumors (NETs) [Citation3,Citation4], but any neoplastic lesion with expression of SSTRs may show increased tracer uptake. Earlier studies with SSTR2 specific octreotide scintigraphy indicated that lymphomas may express SSTRs but at a much lower level compared to NETs. This is likely due to low or variable SSTR subtype expression and density and limited sensitivity of scintigraphic imaging [Citation5–9]. Since imaging with 68Ga-DOTA-conjugated peptide PET/CT greatly improves sensitivity to detection of SSTRs [Citation1,Citation10], a more up-to-date study with PET/CT could help further establish the role of SSTR imaging in lymphomas.
A recent case report presented a patient with a history of bronchial carcinoid whose relapse proved to be diffuse large B-cell lymphoma (DLBCL), which mimicked NET on 68Ga-DOTANOC PET/CT [Citation11]. At the same time a comparable patient was seen in our institution where an indeterminate pancreatic lesion suggested NET based on a positive 68Ga-DOTANOC PET/CT. This lesion did not respond to subcutaneous octreotide therapy and later turned out to be DLBCL (). These two cases prompted us to undertake a prospective study where patients with lymphoma underwent 68Ga-DOTANOC and FDG PET/CT imaging and immunohistochemical analysis (IHC) of tumor SSTR2,3,5 status prior to treatment. We aimed to assess how commonly diverse lymphomas are positive on SSTR imaging and whether reactive rather than tumor cells are responsible for uptake of 68Ga-DOTANOC.
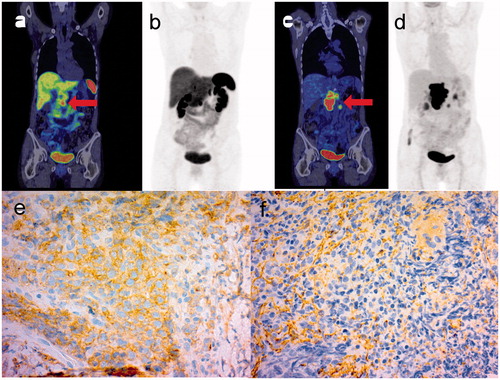
Figure 1. 68Ga-DOTANOC PET/CT (a) and maximum intensity projection (MIP) (b) images with corresponding FDG PET/CT images (c, d) of a patient with DLBCL in the pancreas mimicking NET. The lymphomatous tumor in the head of the pancreas is indicated with an arrowhead. Note that FDG PET/CT was performed six months after the first scan, and progression of lymphoma while the patient was on octreotide treatment is evident. IHC of subtype 2 (e) is positive for the malignant cells, while IHC of subtype 3 (f) shows immunopositivity only in endothelial and connective tissue.
Material and methods
Patients
Twenty-one patients aged between 18 and 80 years (11 male, 10 female, mean age 66 years) with newly diagnosed lymphoma were recruited to this pilot study (). All patients had undergone routine biopsy and a histologically confirmed diagnosis was available at the moment of enrollment. All lymphoma subtypes were included, resulting in five cases of DLBCL (24%), five Hodgkin lymphomas (24%), four follicular lymphomas (19%), three small lymphocytic lymphomas (SLL) (14%), two mantle cell lymphomas (9,5%), one extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) and one anaplastic large cell lymphoma, ALK negative. Exclusion criteria were age under 18 years, pregnancy or lactation, any medical or psychiatric condition that could compromise the subject’s ability to participate in the study, or any other significant disease including liver or renal disease. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1975 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants included in the study, which was approved by the Ethics Committee of the Hospital District of Southwest Finland and by Turku Clinical Research Centre. The study has been registered at ClinicalTrials.gov (NCT02389101).
Table 1. Clinical characteristics and SSTR status of 21 patients with lymphoma.
PET/CT imaging and image analysis
Whole-body 68Ga-DOTANOC and 18F-FDG PET/CT were performed in random order prior to any oncological treatment with either 64-row Discovery STE or VCT (General Electric Medical Systems, Milwaukee, WI, USA) scanner. The imaging protocol for both PET studies was in accordance with the European Association of Nuclear Medicine (EANM) guidelines [Citation1,Citation12] and covered the whole torso from mid-thigh to the base of the skull. The mean injected activity of 68Ga-DOTANOC was 126 MBq (109–143) and that of FDG 297 MBq (218–411). The start of acquisition was 60 min after injection in all cases. The acquisition time was 4 min per bed position in 68Ga-DOTANOC PET/CT and 2 or 3 min per bed position in FDG PET/CT depending on the scanner used. Low-dose CT was used for attenuation correction. The median time between the two PET studies was nine days (1–29). One patient (No. 16) developed disruptive itching as a B-symptom after 68Ga-DOTANOC imaging and received Prednisone 10 mg once per day for three days before FDG PET/CT and 40 mg Prednisolone just before FDG PET/CT.
PET images were reconstructed in 3D mode and 128 × 128 matrix size using an ML-OSEM reconstruction algorithm. Two nuclear medicine specialists blinded to the histological results evaluated the images using an ADW 4.6 workstation (General Electric Medical Systems, Milwaukee, WI, USA). Maximum standardized uptake values (SUVmax) were determined from 14 lymph node and two extranodal regions with the highest uptake in each patient. The SUVmax values were corrected for body weight and injected dose. Lesions were then graded with the Deauville score [Citation1–5] on FDG PET/CT and modified Krenning score (0–4) on 68Ga-DOTANOC PET/CT () [Citation13,Citation14]. Lymphomas were graded as FDG-positive if the Deauville score was ≥3. Uptake of 68Ga-DOTANOC was regarded as positive if the modified Krenning score was ≥2. We recognize that Deauville scoring is primarily validated for evaluation of interim FDG PET of patients receiving chemotherapy, but adopted it for baseline scans owing to its convenience for the study.
Table 2. Criteria for visual grading of pathological tracer uptake on FDG PET/CT and 68Ga-DOTANOC PET/CT.
Histological analysis
SSTR2,3,5 status was analyzed from tissue samples obtained from routine biopsies by a pathologist specialized in lymphomas and blinded to the PET/CT findings. Formalin-fixed paraffin-embedded tumor tissues were sectioned at 3 μm and used for analysis. Primary antibodies used for IHC were SSTR2/UMB1 (dilution 1:1000), SSTR3/UMB5 (dilution 1:2000) and SSTR5/UMB4 (dilution 1:500) (Abcam, Cambridge, UK). Staining was done with either Ventana Benchmark XT Autostainer (UMB1-staining) with ultraVIEW Universal Detection Kit (Ventana, Strasbourg, France), or Labvision Autostainer with Envision secondary antibody (Dako, Glostrup, Denmark).
Results
PET/CT imaging
On visual analysis, 20 of 21 (95%) patients had FDG-positive and 13 of 21 (62%) had 68Ga-DOTANOC-positive lymphoma (). Patient no. 14 with SLL had the only FDG-negative lymphoma. Tracer uptake expressed as median SUVmax of all positive lesions and highest SUVmax of all 68Ga-DOTANOC-positive lymphomas is summarized in . 68Ga-DOTANOC-positivity was seen predominantly in lymph nodes that were invariably also FDG positive with only one exception (patient no. 4), where four lymph nodes were 68Ga-DOTANOC-positive but only two of them were FDG positive. There was no clear correlation between SUVmax in 68Ga-DOTANOC and FDG positive lymph nodes. The highest uptake of 68Ga-DOTANOC was seen in a patient with Hodgkin’s lymphoma of the nodular sclerosis subtype (no. 19) whose median SUVmax of the positive lesions was 9.8. Another patient (no. 3) with DLBCL (GCB subtype) had a respective median SUVmax of 9.7. This patient was the only case with conspicuous 68Ga-DOTANOC-positive extranodal lesions ().
Figure 2. 68Ga-DOTANOC PET/CT (a) and maximum intensity projection (MIP) (b) whole body images of a patient with DLBCL of germinal-cell type, showing a large left axillary nodal and upper arm extranodal lesion and multiple other nodal lesions on both sides of the diaphragm. The corresponding FDG PET/CT images (c, d) show a concordant pattern of tracer uptake. IHC of subtype 2 (e) is strongly positive in tumor cells while IHC of subtype 3 (f) shows staining mainly in the endothelial linings of veins.
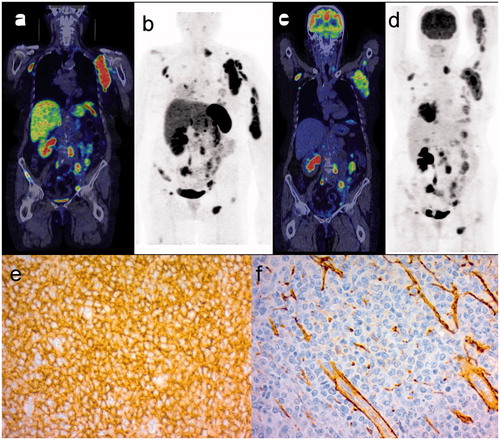
Table 3. Comparison between uptake of 68Ga-DOTANOC and FDG in lymphomatous lesions in 13 patients with positive findings at 68Ga-DOTANOC PET/CT.
In the majority of patients extranodal lesions were 68Ga-DOTANOC negative, although faint or moderate uptake in bone and bowel lesions was evident in a total of six patients, of whom four had a Krenning score of ≥2. Upon visual assessment, two patients presented with uptake of 68Ga-DOTANOC in a non-neoplastic lesion. This interpretation was based on international guidelines, correlation with anatomic findings and previously recognized pitfalls in 68Ga-DOTANOC imaging [Citation1,Citation14]. In line with this, a pelvic abscess and possible pulmonary inflammation were detected in a patient with DLBCL (no. 4) and a patient with SLL (no. 14), respectively. A third patient (no. 18) showed clear uptake in the head of the pancreas, eventually interpreted as a rare cystic form of NET since the corresponding MRI suggested a cystic tumor at the same site. The patient’s serum CgA was 2.3 nmol/l and a biopsy was not considered, since the patient currently remains symptom-free of a low-grade NET.
Somatostatin receptor immunohistochemistry (IHC)
SSTR2 immunopositivity was demonstrated consistently in macrophages, follicular dendritic cells and endothelial cells of the veins. All four patients with follicular lymphomas showed SSTR2 immunopositivity in neoplastic follicles (mainly in dendritic cells), but also scattered positivity in the malignant B-cells. Of these patients, two had a positive 68Ga-DOTANOC PET/CT, which likely was due to tracer uptake in non-neoplastic cells. By contrast, patient no. 3 with DLBCL showed strong SSTR2 immunopositivity in malignant B-cells in agreement with the 68Ga-DOTANOC PET/CT finding (). Also the four other DLBLCs had a positive PET/CT but their SSTR2 immunopositivity was limited to venous endothelial cells, which probably contributed to the positive PET/CT result since DLBCL has abundant vessel formation associated with neoplastic transformation.
An observation of note was that in all four Hodgkin’s lymphoma patients with successful SSTR2 IHC, the cell membrane of neoplastic Reed–Sternberg (R–S) and Hodgkin cells was SSTR2 positive (). Interestingly, immunopositivity in R-S cells did not always translate into a positive 68Ga-DOTANOC PET/CT, since only half of these patients had positive findings on SSTR imaging. This is not a surprise given that R–S cells are scattered and typically low in number amid a group of lymphocytes and other reactive cells present in lymphomatous tissue. Unfortunately, the fifth case of Hodgkin’s lymphoma with conspicuous and high uptake of 68Ga-DOTANOC in lymph nodes (no. 19) had unsuccessful SSTR2 IHC.
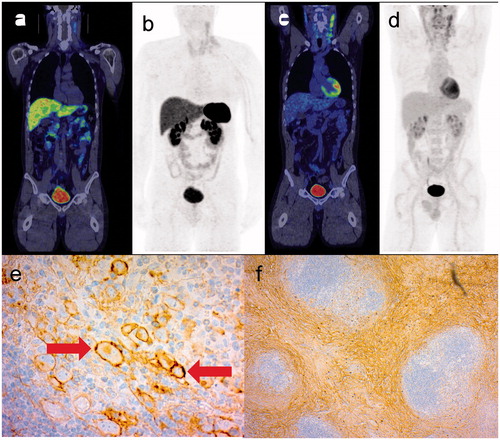
Figure 3. 68Ga-DOTANOC PET/CT (a) and maximum intensity projection (MIP) (b) whole body images of a patient with Hodgkin’s lymphoma of nodular sclerosis type showing multiple positive left neck nodes and concurrent findings on corresponding FDG PET/CT images (c, d). IHC of subtype 2 (e) is positive for the cell membrane of the neoplastic Reed–Sternberg cells (arrows), while IHC of subtype 3 (f) shows staining of collagen bands.
SSTR3 was mainly negative in the malignant cells of all lymphoma subtypes, except for the abovementioned Hodgkin’s lymphoma patient (no. 19) who showed SSTR3 immunopositivity in the cytoplasm of R–S cells. Some SSTR3 immunopositivity was observed in macrophages, mast cells and endothelial cells, which could have some impact on uptake of 68Ga-DOTANOC especially in the five DLBCLs where venous endothelial linings stained positive for SSTR3. Another concordant finding was that in the three Hodgkin lymphomas of the nodular sclerosis subtype, the collagen bands characteristic of this disease showed SSTR3 immunopositivity possibly contributing to positive 68Ga-DOTANOC PET/CT together with expression of SSTR2 ().
SSTR5 was positive in the malignant cells of a patient with SLL (no. 12) and a patient with Hodgkin’s lymphoma of the nodular sclerosis subtype (no. 20). SSTR5 was also interpreted as positive in patient nos. 1 and 19, but their IHC was regarded as unreliable even after repeated analysis. Therefore, their SSTR5 status was left as uncertain. In all other patients, SSTR5 IHC was negative in the malignant cells.
Discussion
In this pilot study, more than half of lymphomas (13 of 21; 62%) showed uptake of 68Ga-DOTANOC. The highest uptake of 68Ga-DOTANOC was seen in single patients with Hodgkin’s lymphoma of the nodular sclerosis subtype and DLBCL. Both patients showed strong SSTR2 immunopositivity in their tumor cells. In other DOTANOC-positive lymphomas, SSTR2 immunopositivity was seen predominantly in endothelial and dendritic cells and the follicular centers of lymph nodes.
SSTRs are expressed in a wide variety of tumors but their significance is not established, with the exception of NETs. The majority of NETs have an abundance of SSTRs, which renders them 68Ga-DOTANOC PET/CT positive and amenable to peptide receptor radionuclide therapy (PRRT) [Citation1,Citation4,Citation15,Citation16]. In a recently presented multicenter phase III study (NETTER-1), PRRT with 177Lu-DOTATATE was associated with improved progression-free survival and possible survival benefit in patients having advanced midgut NETs progressing after somatostatin analog therapy [Citation17]. These highly successful clinical findings have formed a proof-of-concept for theranostic imaging and attract evaluation of SSTR imaging in other tumor types such as meningiomas and thyroid cancer [Citation18,Citation19]. Lymphomas in particular have been shown to express SSTRs [Citation5,Citation7,Citation20,Citation21], but their clinical value has been questioned, since more recent studies have concluded that the expression profile of lymphomas may be inadequate for SSTR-based imaging and treatment [Citation6,Citation9,Citation22].
In spite of this we were encouraged by a recent case report [Citation11] and single experience from our own institution to undertake a prospective study where SSTR status was evaluated in vivo and in vitro by 68Ga-DOTANOC PET/CT and immunohistochemical analysis in patients with newly diagnosed lymphoma. Our results showed clear SSTR2 positivity in IHC in malignant lymphoma cells in one DLBCL and in all Hodgkin’s lymphoma patients, and SSTR3 positivity in collagen bands of Hodgkin’s lymphoma of the nodular sclerosis subtype. These immunohistochemical findings were concordant with 68Ga-DOTANOC PET/CT where the SSTR2 positive DLBCL and all Hodgkin’s lymphomas of the nodular sclerosis subtype showed clear tracer uptake. In other lymphomas, the malignant cells were not as clearly positive in any SSTR IHC, which indicates that most of the positive 68Ga-DOTANOC PET/CT findings were due to uptake in non-neoplastic cells such as macrophages and dendritic cells. Also, there was no relationship between positive uptake expressed as SUVmax and SSTR IHC in tumor cells, suggesting that non-neoplastic cells in lymphomatous lesions had considerable impact on imaging findings. The possibility of SSTR imaging positivity is of major importance for differential diagnosis, especially where NET is suspected and a representative biopsy is not available.
The absolute uptake – be it specific or nonspecific – remains on average at a substantially lower lever compared to NETs. Hence, PRRT with octreotide analogs does not intuitively seem to be an attractive method to treat lymphomas, and this has been suggested in an earlier study as well [Citation22]. However, since lymphomas are highly sensitive to radiation we do not rule out SSTR-based PRRT in palliative treatment of well-selected cases. We propose further investigation of this approach based on the well-known possibility of a long-term response to low-dose external beam radiation in some indolent lymphomas [Citation23].
Our study has some limitations. First, we do not have histological confirmation of all 68Ga-DOTANOC positive lesions, but rather used FDG PET/CT as a reference where increased uptake (Deauville score ≥3), coupled with a suitable anatomic location and morphologically suspicious mass or lesion, was regarded as a tumor. Notwithstanding, it is possible that some false positive lesions were included in our evaluation. Like FDG, 68Ga-DOTANOC is excreted via the urinary system. Other organs with physiological uptake include the pancreas, liver, adrenal glands, thyroid, spleen and stomach. Possible false positive findings include focal physiological uptake in the pancreas, accessory spleen, osteoblastic activity and inflammation or infection, which should be carefully noted in the interpretation of 68Ga-DOTANOC PET/CT [Citation1,Citation14]. We believe that these pitfalls do not affect our general conclusions since concordant findings in IHC confirmed uptake in lymphomatous lesions, although it was not specific to tumor cells but often occurred in the adjacent immunoreactive cells or endothelial linings. Second, our method was unable to reliably evaluate SSTR5 status in two out of four SSTR5-positive patients in IHC, which leaves some uncertainty as to the impact of SSTR5 on imaging results. The most important receptor subtype seems to be SSTR2, and to some extent SSTR3, as shown also in earlier studies [Citation22,Citation24]. Third, we included all lymphoma subtypes in this pilot study, which resulted in a small number of patients in each histological subtype. Hence, further research is needed to investigate which lymphoma subtypes are most likely to express SSTRs in the transformation of immune cells to neoplasia. Whether SSTR expression in neoplastic transformation to lymphoma is an early or late event needs clarification, and it would be important to relate SSTR status to outcome. We have begun a study into this in collaboration with a local university-based Biobank.
Conclusions
Some malignant lymphomas show DOTANOC uptake on PET/CT, which is due to SSTR expression in e.g. Reed-Sternberg cells in Hodgkin’s lymphoma or adjacent cells contributing to the development of lymphomatous lesions, notably in DLBCL. While EANM guidelines suggest that lymphomas have low SSTR expression, our results show that there are exceptions. This is a potential source of error in the differential diagnosis of NETs, while on the other hand it may offer a possibility to develop treatments of lymphomas targeting SSTR. While lymphomas are very radiosensitive, high expression of SSTR in Hodgkin’s lymphoma and DLBCL may even pave the way to developing a palliative treatment option for these lymphoma subtypes.
Metabolite_analysis.docx
Download MS Word (397.3 KB)Acknowledgments
We thank the staffs of Turku PET Centre and the Department of Oncology and Radiotherapy, Turku University Hospital, for their practical assistance in conducting this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Virgolini I, Ambrosini V, Bomanji JB, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37:2004–2010.
- Wild D, Schmitt JS, Ginj M, et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging. 2003;30:1338–1347.
- Kaemmerer D, Peter L, Lupp A, et al. Molecular imaging with (6)(8)Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:1659–1668.
- Ambrosini V, Campana D, Bodei L, et al. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. J Nucl Med. 2010;51:669–673.
- Vanhagen PM, Krenning EP, Reubi JC, et al. Somatostatin analogue scintigraphy of malignant lymphomas. Br J Haematol. 1993;83:75–79.
- Ferone D, Semino C, Boschetti M, et al. Initial staging of lymphoma with octreotide and other receptor imaging agents. Semin Nucl Med. 2005;35:176–185.
- Lugtenburg PJ, Krenning EP, Valkema R, et al. Somatostatin receptor scintigraphy useful in stage I-II Hodgkin's disease: more extended disease identified. Br J Haematol. 2001;112:936–944.
- Kwekkeboom DJ, Krenning EP. Somatostatin receptor imaging. Semin Nucl Med. 2002;32:84–91.
- Ivancevic V, Wormann B, Nauck C, et al. Somatostatin receptor scintigraphy in the staging of lymphomas. Leuk Lymphoma. 1997;26:107–114.
- Antunes P, Ginj M, Zhang H, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34:982–993.
- Jain S, Sharma P, Dhull VS, et al. Lymphoma as a second malignancy in a patient with neuroendocrine tumor: mimicking dedifferentiation on dual-tracer PET/CT with 68Ga-DOTANOC and 18F-FDG. Clin Nucl Med. 2014;39:358–359.
- Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354.
- Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. JCO. 2014;32:3048–3058.
- Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35:500–516.
- Frilling A, Weber F, Saner F, et al. Treatment with (90)Y- and (177)Lu-DOTATOC in patients with metastatic neuroendocrine tumors. Surgery. 2006;140:968–976. discussion 976-7.
- Kam BL, Teunissen JJ, Krenning EP, et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39:S103–S112.
- NETTER-1 phase III in patients with midgut neuroendocrine tumors treated with 177Lu-DOTATATE: efficacy and safety results. Clin Adv Hematol Oncol. 2016;14:8–9.
- Woelfl S, Bogner S, Huber H, et al. Expression of somatostatin receptor subtype 2 and subtype 5 in thyroid malignancies. Nuklearmedizin. 2014;53:179–185.
- Silva CB, Ongaratti BR, Trott G, et al. Expression of somatostatin receptors (SSTR1-SSTR5) in meningiomas and its clinicopathological significance. Int J Clin Exp Pathol. 2015;8:13185–13192.
- Reubi JC, Waser B, van Hagen M, et al. In vitro and in vivo detection of somatostatin receptors in human malignant lymphomas. Int J Cancer. 1992;50:895–900.
- Ferone D, Hofland LJ, Colao A, et al. Neuroendocrine aspects of immunolymphoproliferative diseases. Ann Oncol. 2001;12:S125–S130.
- Dalm VA, Hofland LJ, Mooy CM, et al. Somatostatin receptors in malignant lymphomas: targets for radiotherapy?. J Nucl Med. 2004;45:8–16.
- Chan EK, Fung S, Gospodarowicz M, et al. Palliation by low-dose local radiation therapy for indolent non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2011;81:e781–e786.
- Reubi JC, Schaer JC, Waser B, et al. Expression and localization of somatostatin receptor SSTR1, SSTR2, and SSTR3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res. 1994;54:3455–3459.
