Abstract
Purpose: To better estimate relative biological effectiveness (RBE) in therapeutic proton beams by using a modeled approach, in order to improve their clinical safety and effectiveness.
Introduction: Concerns exist about the 1.1 RBE used in proton therapy, since it may lead to unintentional over- and under-dosage in patients and so lead to unexpected clinical outcomes. Late reacting normal tissues (with low α/β values), might be overdosed if RBE >1.1; very radiosensitive tumors (with high α/β), might be under-dosed if RBE <1.1. Some physicists recommend ignoring RBE in favor of a LET × dose product to predict effects.
Material and methods: Extensive linear-quadratic based modeling is scaled between a standard hospital megavoltage photon reference radiation (low LET of 0.22 keV μm−1) α and β values and their values at higher LETs, representative of the middle and end of the SOBPs. A previously published energy-efficiency model provide RBE estimates for different α/β (2–27 Gy). The concept of using a LET × dose product is assessed by comparing it with surviving fraction and the equivalent dose in 2 Gy fractions (EQD-2).
Results: Low α/β value biosystems have the widest RBE ranges with dose per fraction changes and increasing LET, often above 1.1 even within the SOBP LET range, with lower values at higher dose per fraction. Highly radiosensitive tumors (α/β 10–27 Gy) have the lowest RBEs, often below 1.1, and are not fraction-sensitive. RBE’s generally increase with LET, so curtailment of LET in normal tissues is important. The LET × dose product is insufficiently discriminating when compared with surviving fraction and biological effective dose (BED) or EQD-2.
Conclusions: An overall research framework is suggested. Proton therapy advantages will only be fully realized if reasonably correct RBE values are used.
Introduction
Many countries are either extending their existing proton facilities or embarking on proton and ion beam treatments for the first time. Such decisions are based on the potential advantages of reduced collateral radiation to normal tissues while delivering suitable tumor doses. There remain concerns about enhanced biological effects in normal tissues due to increased clustering of DNA damage as a consequence of increased linear energy transfer (LET) that occur in Bragg peak regions [Citation1]. Relative biological effectiveness (RBE) is used to compensate for raised LET effects, such that RBE-guided dose reductions are necessary to respect the dose constraints in normal tissues that are either within or without the primary target volumes. Normal tissues are inevitably treated in the ICRU approved volumes that surround tumors [Citation2], and these volumes are often larger than the tumor volume itself. Late reacting normal tissues are known to have high photon dose per fraction sensitivities that differ markedly from acute reacting tissues: this will be reflected in their RBE ranges. It is important to remember that RBE is defined as the reference radiation dose divided by the higher LET particle dose, and for late reacting tissues the reference radiation (numerator) dose will change to a greater extent than would be the case for an acute reacting tissue. Also, because of the fact that high LET radiations sensitize α to a greater extent than β, at low doses (where α cell killing predominates) the RBE will be higher in late reacting tissues (with low α/β ratios) than in acute reacting tissues, which already have a high α/β ratio and subsequent increments are not so influential [Citation2–4]. The volume of tissue that could be ‘slightly overdosed’ may not be so clinically important in a parallel organized and/or self-replicating tissue, but in serially organized tissues with important function, great care needs to be taken on the use of RBE values. The present global use of a generic proton RBE of 1.1 in all tissue types and at all doses and LET values has been criticized for a number of valid reasons [Citation2–10], yet continues to be used.
Although there may be greater consensus that LET values above the averaged values found in SOBP (1–2 keV μm−1), should be avoided in critical normal tissues, values as high as 8–10 keV μm−1 can be found with scanned proton beams [Citation11]. Even if this occurs in relatively low dose areas, they may have clinical significance. For example, if an organ-at-risk (OAR) structure has a tolerance value of say 50 Gy in 25 fractions and receives 65% of the PTV dose of 72 Gy-Eq (RBE = 1.1), then it is assumed that the OAR receives 50 × 0.65 = 46.8 Gy. If the relevant LET results in a dose-per-fraction adjusted RBE of say 1.25 (rather than 1.1), then the actual dose equivalent will be (1.25/1.1) × 46.8 = 53.2 Gy, which exceeds tolerance. The situation would be worse if the RBE was to be 1.3: then, the dose equivalent would be (1.3/1.1) × 46.8 = 55.3 Gy. The clinical situation would be further exacerbated due to much higher RBE's for LET values of 6–10 keV μm−1 [Citation2].
There remains a significant debate about the RBE values in SOBPs. Some regard this as being conveniently 1.1, but this figure is appropriate only for early reacting tissues, since the assays used to achieve this value were of this type. Also, the high dose ranges used as well as only linear fitting analysis is open to criticism as well as the LET of the reference radiation in many of the experiments used [Citation4].
Some authorities have suggested (in international meetings) that the product of LET × dose will provide an estimate of risk in a treatment plan. Although this is attractive due to its simplicity, the product cannot accurately reflect the risk associated with RBE. This is because LET is, in most instances, directly proportional to RBE, but increasing the dose per fraction reduces RBE. In this way, dose and LET can act in different numerical directions within their product. Dimensional analysis of the product does not provide a scientifically meaningful product (see mathematical appendix in Supplementary files).
Our knowledge of proton LET-RBE interactions remains patchy, based on experiments containing relatively few data points, undertaken in different laboratories and using a limited range of cell lines. Several predictive models exist, but none have been thoroughly verified. Most link LET to RBE using a function of the reference radiation α/β [Citation5,Citation6,Citation9,Citation12,Citation13], but the model used in this report uses separate functions for α and β, based on a common LETU value (the LET at which the RBE is greatest), which preserves the LET–RBE symmetry with increasing dose per fraction [Citation2,Citation3] due to the reduction in the dominance of α over β related cell kill. The problem associated with using a function of α/β only can be seen by the fact that RBE can be split into two components, the RBEmax (=αH/αL), which is the RBE at very low dose, and RBEmin (=√βH/√βL), the RBE at high dose, and where subscripts L and H refer to the reference radiation (low LET) and the high LET value respectively. From these definitions, with some algebraic re-arrangement it follows that RBEmax and RBEmin are respectively proportional to 1/(α/β)L and √(α/β)L, which oppose each other. Although the RBEmax relationship only is used in most models, there may be increasing errors in RBE estimation with larger doses per fraction, since RBEmin then becomes more important. For these reasons separate treatment of α and β is recommended if full dose ranges are to be considered.
Another issue is that there are no general requirements which must be fulfilled before any such model is completely acceptable. This is in marked contrast to say the requirements in bacteriology such as Koch's postulates, which rigorously define the criteria by which an infectious disease may be confirmed [Citation14]. The following criteria are suggested for high LET studies:
The increase in α and β with LET should be known per unit increase in LET, to the value of LETU, where the radiosensitivities begin to decrease, when a further slop or curvature should be estimated.
The maximum values, αU and βU at LETU should be determined and related to the reference radiation low LET α and β.
The position of LETU should be confirmed for different cellular systems for each ion beam.
The value of RBE at LETU should be determined for several dose levels and the overall symmetry of the LET–RBE–dose relationship determined.
Such information, if accurately determined, would allow proton or ion BED iso-effect calculations to be done with greater confidence. At present it is only possible to use modeling in a tentative way to provide clinical guidance.
This article provides some insight into the uncertainties associated with LET–RBE relationships, presented as testable hypotheses based on the phenomena that have been demonstrated in the past. It also considers what approaches may are necessary to provide rigorous and definitive data upon which more robust predictive models can be based and the potential clinical implications of such knowledge.
Methods
A reference radiation LET value (LETC) of 0.22 keV μm−1 is used in all calculations [Citation15,Citation16] as this seems to be appropriate for megavoltage irradiation used in the clinic and upon which most tolerance doses are based. The LET is assumed to be the averaged value in a relevant clinical volume or radiological voxel [Citation11]. The equations given in references 2 and 3 are used to scale radio-sensitivities from the above reference radiation value (rather than 1 keV μm−1 used in previous publications [Citation2,Citation3], which was typical of many radiobiological experiments using orthovoltage or low voltage photons as the reference radiation), combined with other equations which provide a saturation (or slope reduction) effect for the increment in α and β with LET up to their maximum values. The scaling of α and β between the reference LET value of LETC and their ultimate values at the turnover point of the LET–RBE relationship, where LET is defined as LETU, which appears to be specific for each ion beam species and around 30 keV μm−1 for protons (readers should consult references 2 and 3 in detail). The relevant equations are summarized in the Supplementary information files.
The estimated RBE’s can then be plotted for different low LET baseline α/β values. The assumptions made for the simpler estimates are that the β parameter is relatively stable at 0.03 Gy−1 for all α/β values (varied between 2 and 27 Gy). This is to reflect α/β in late reacting central nervous system tissue, as the lowest ratio, and then higher values which reflect increasingly radiosensitive tumor systems.
The sensitivity of the RBE estimations to the assumed position of LETU (the LET at which the radio-sensitivities are maximal), are obtained by graphical methods for LET values below and above the 30.5 keV μm−1, as used in this study as obtained by Belli et al. [Citation17].
An alternative system using stochastic estimates is obtained by assuming mean α values of 0.07 Gy−1, and α/β of 2 and 3 Gy for late-reacting normal tissues, but α values of 0.275, 0.375 and 0.55 Gy−1 for tumors with α/β ratios of respectively 4.5, 10 and 25 Gy (the β parameters are then found by using these ratios). In all cases a standard deviation of 10% of the mean was used in Mathematica (Champaign, IL, USA) random sampling procedures using 1000 samples, which provide the mean RBE and the 1 and 99% quantile ranges. The RBE values are estimated as shown in the appendix for 1, 1.5, 2 and 4 keV μm−1 and a range of dose values.
In a separate exercise, the LET × dose product is plotted as ‘box and whisker’ plots with surviving fraction (SF) for helium and carbon ions using the data sets of Barendsen [Citation18] and Weyrather et al. [Citation19], using only LET values which are less than LETU, the value at the turnover point of the LET–RBE relationship.
Also, a simulation of the same product plotted against and EQD-2 (the equivalent dose using 2 Gy fractions) for protons, using the α and β values obtained by Britten et al. [Citation7] at Bloomington. The high LET BED is obtained using the RBEmax and RBEmin concepts (see Appendix). Plots of BED (which is proportional to SF) and EQD-2 9 the equivalent dose in 2 Gy fractions) are also generated for two different α/β values (2 and 10 Gy), for two proton LET variants (1 and 2 keV μm−1), and the reference radiation photon LET of 0.22 keV μm−1.
Results
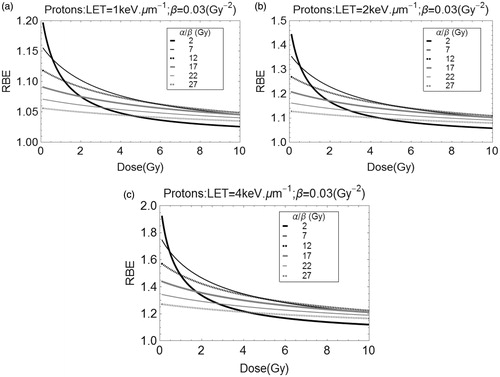
) show estimated RBE changes with dose per fraction for a range of α/β for specific LET values. It can be seen that the highest RBE at low dose is found for α/β of 2 Gy (representing CNS tissues), but since this α/β will cause a large change in the reference radiation iso-effective dose with increasing dose per fraction, the RBE falls below values found for tissues or tumors with higher α/β characteristics. This is especially important when the LET increases from 1 to 2 keV μm−1, since the values may significantly exceed 1.1 at low dose, and increase further with LET beyond 2 keV μm−1. The prospects for using hypo-fractionated proton beams become more attractive for reduction of late tissue morbidity, due to the fall in RBE noted.
Of further concern are the very low RBEs found for very high α/β tissue systems (such as those found in radiosensitive conditions such as lymphomas and in childhood tumors). Since these RBE's are smaller than 1.1, tumor under-dosage could occur using standard proton therapy practice.
These concerns are also confirmed by inspection of Tables 1–5 (see supplementary files) which use different radiobiological assumptions, with ranges of RBE which are perhaps more instructive to clinicians and physicists. These tables also include some intermediate α/β ratios more typical of slow-growing and more radio-resistant tumors, such as chordoma, prostate and breast. Ideally, such tables should be consulted where there is clinical concern.
The sensitivity of the model to the value of LETU can be seen in the Supplementary file as figure S1(a and b) for the lowest α/β ratio of 2 Gy used in the study, which represents the worst possible case where RBE changes most with dose per fraction. A change in LETU of 5 keV μm−1 provides only a 1–2% change in RBE, for irradiations assumed to be at 1 and 2 keV μm−1.
The potential errors of using the LET × dose product is shown in the Supplementary files as figures S2(a–c), where there is clear overlap in the ion beam product ranges for markedly different values of clonogenic surviving fraction (SF). It is self-evident that the product cannot be sufficiently discriminating for more subtle changes in SF than those shown. Also, the proton plot shown in of the main text reveals a wide range of EQD-2(Gy) variation, falling within a large trapezoid phase-space in the case of parameters obtained from one human cell line. Although the EQD-2 does increases with the LET–dose product, the variation encountered seems to be counter-productive and unreliable.
Figure 2. Phase space of equivalent dose in 2 Gy fractions (EQD-2), derived from BED values, plotted against the dose × LET product for proton-irradiated hep-2 cell data of Britten et al. [Citation7].
![Figure 2. Phase space of equivalent dose in 2 Gy fractions (EQD-2), derived from BED values, plotted against the dose × LET product for proton-irradiated hep-2 cell data of Britten et al. [Citation7].](/cms/asset/dfcaf237-47f2-4a95-bfc9-9158a7cdb1de/ionc_a_1343496_f0002_b.jpg)
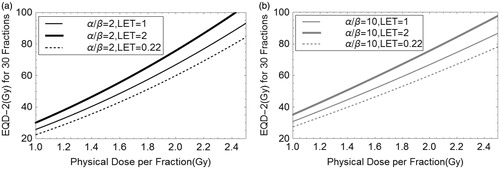
For potential clinical significance, shows the increments in the thirty fraction EQD-2 with dose per fraction for LET’s of 0.22 (megavoltage photons), 1 and 2 keV μm−1 (protons) for α/β values of 2 and 10 Gy. Between photons and protons with LET of 2 keV μm−1 there are around 30 Gy and 15 Gy differences in BED for α/β values of 2 and 10 Gy, respectively. When the dose per fraction is 2 Gy, the LET increments correspond to an additional 8–9 Gy (for α/β = 2 Gy), and increments of around 7–8 Gy for α/β = 10 Gy. These are significant changes which might produce additional side effects in critical normal tissues, but additional cell killing effects in tumors.
Discussion
The above results indicate that great care must be taken in the medical prescription of proton therapy. Particularly important will be the requirement to:
Produce 3-D LET maps as well as dose maps,
Generate estimated RBE values in critical normal tissues and in radio-resistant tumors, using reasonable models.
Adjust the tolerance doses of critical structures according to these RBE values.
Maintain the maximum tumor biologically effective dose (BED) or EQD-2 with acceptable BED values below tolerances wherever possible in normal tissues.
There are clinical concerns among those who have referred patients for proton therapy and have noted either greater than expected serious toxicities or disappointing tumor control. The report of 12.8% high grade toxicity (mainly temporal lobe necrosis and visual loss in skull base tumors in a specialized center is of concern [Citation20]. Such a serious neurological complication incidence would be considered unacceptable by most radiation oncologists who use photons. A further report on pediatric brainstem toxicity raises similar concerns [Citation21]. Although many physicists will argue that range variations may account for at least some of these effects, there is probably greater variation in dose due to RBE uncertainties at the present time, as shown in the ranges of RBE estimated in this report.
The LET × Dose product will not be so helpful compared with reliable BED estimations over a wide product range, although small product changes will correlate with BED changes in the same direction (better or worse), and so could be used only for very simplistic dose plan comparisons containing only minor deviations. It is only by using LET, RBE and dose that reasonable BED or EQD-2 estimations can be generated for clinical assessment purposes. If the RBE concept did not already exist, it would be necessary to invent it, as it is the best way of translating LET changes into dose modifications that can be compared with the existing photon based radiotherapy knowledge base.
There is considerable scope for further research, starting with the four criteria mentioned in the introduction. The accuracy of the present RBE estimations depend upon relatively limited data sets and assumptions, especially in proton therapy [Citation22]. If these were to be strengthened by further research using larger sized experiments, there would be greater confidence in the modeling parameters used. Whether suggestions for an international cooperative laboratory for RBE and other particle beam studies [Citation23,Citation24] can be achieved at CERN (European Centre for Nuclear Research, Geneva), is undecided.
In addition, if in vivo research proves to be too difficult or expensive to provide comprehensive solutions, then observational data in patients already treated may provide further evidence of RBE effects, for example by noting functional imaging tissue changes and comparing these with what can be expected using megavoltage photons.
Design of clinical trials is also important and ideally trials should include two variants of photon dose and two variants of proton dose in order to provide sufficient data that can be used for further optimization and analysis of RBE effects for future applications. By randomizing the RBE assumption made (either constant or variable) then the randomization process would separate physics dose placement issues from RBE causes of toxicity.
There is much to be gained by taking RBE more seriously in proton therapy.
IONC_A_1343496_Supplementary_Information.zip
Download Zip (1.6 MB)Acknowledgments
The author is grateful for discussions of RBE with many scientists and clinicians, but especially RG Dale and JH Hopewell. Also for the award of Guest Professor at CERN (2015–2016).
Disclosure statement
None apart from the generous travel expenses provided by IBA (Louvain) for attending the proton therapy expert workshop in Dresden during 2016.
References
- Anderson RM, Stevens DL, Sumption ND, et al. Effect of linear energy transfer (LET) on the complexity of alpha-particle-induced chromosome aberrations in human CD34+ cells. Radiat Res. 2007;167:541–550.
- Jones B. Towards achieving the full clinical potential of proton therapy by inclusion of LET and RBE models. Cancers (Basel). 2015;7:460–480.
- Jones B. A simpler energy transfer efficiency model to predict relative biological effect (RBE) for protons and heavier ions. Front Oncol. 2015;5:184. DOI:10.3389/fonc.2015.00184. Erratum in: Front Oncol. 2016;6:32.
- Jones B. Why RBE must be a variable and not a constant in proton therapy. Br J Radiol. 2016;89:20160116. DOI:10.1259/bjr.20160116
- Wilkens JJ, Oelfke U. A phenomenological model for the relative biological effectiveness in therapeutic proton beams. Phys Med Biol. 2004;49:2811–2825.
- Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–R472.
- Britten RA, Nazaryan V, Davis LK, et al. Variations in the RBE for cell killing along the depth-dose profile of a modulated proton therapy beam. Radiat Res. 2013;179:21–28.
- Marshall TI, Chaudhary P, Michaelidesová A, et al. Investigating the implications of a variable RBE on proton dose fractionation across a clinical pencil beam scanned spread-out Bragg peak. Int J Radiat Oncol Biol Phys. 2016;95:70–77.
- Wedenberg M. Toma-Dasu Disregarding RBE variation in treatment plan comparison may lead to bias in favor of proton plans. Med Phys. 2014;41:091706. DOI:10.1118/1.4892930
- Tommasino F, Durante M. Proton radiobiology. Cancers (Basel). 2015;7:353–381.
- Grassberger C, Trofimov A, Lomax A, et al. Variations in linear energy transfer within clinical proton therapy fields and the potential for biological treatment planning. Int J Radiat Oncol Biol Phys. 2011;80:1559.
- Giovannini G, Böhlen T, Cabal G, et al. Variable RBE in proton therapy: comparison of different model predictions and their influence on clinical-like scenarios. Radiat Oncol. 2016;11:68. DOI:10.1186/s13014-016-0642-6.
- Wedenberg M, Lind BK, Hårdemark B. A model for the relative biological effectiveness of protons: the tissue specific parameter α/β of photons is a predictor for the sensitivity to LET changes. Acta Oncol. 2013;52:580–588.
- Fredericks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev. 1996;9:18–33.
- Hall EJ. Radiobiology for the radiologist. 4th ed. Philadelphia: JB Lippincott; 1994. p. 160.
- ICRU Report 16, (International Commission on Radiation Units & Measurements). Linear energy transfer. Bethesda, USA: ICRU; 1970.
- Belli M, Bettega D, Calzolari P, et al. Inactivation of human normal and tumour cells irradiated with low energy protons. Int J Radiat Biol. 2000;76:831–839.
- Barendsen GW. Responses of cultured cells, tumours and normal tissues to radiations of different linear energy transfer. Curr Topics Radiat Res Q. 1968;4:293–356.
- Weyrather WK, Ritter S, Scholz M, et al. RBE for carbon track-segment irradiation in cell lines of differing repair capacity. Int J Radiat Biol. 1999;75:1357–1364.
- Weber DC, Malyapa R, Albertini F, et al. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120:169–174.
- Peeler CR, Mirkovic D, Titt U, et al. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol. 2016;121:395–401.
- Sørensen BS, Overgaard J, Bassler N. In vitro RBE-LET dependence for multiple particle types. Acta Oncol. 2011;50:757–762.
- Holzscheiter MH, Bassler N, Dosanjh M, et al. A community call for a dedicated radiobiological research facility to support particle beam cancer therapy. Radiother Oncol. 2012;105:1–3.
- Dosanjh M, Jones B, Myers S. A possible biomedical facility at CERN. Br J Radiol. 2013;86:1025–1029.