Abstract
Background: To evaluate the outcome of patients affected by a single isolated body metastasis treated with stereotactic body radiotherapy (SBRT).
Material and methods: Seven-eight patients were treated with SBRT for isolated body metastasis. The most frequent primary tumor was prostate cancer (28.2%), followed by colorectal cancer (23.1%) and lung cancer (20.5%). Median age at diagnosis of oligometastatic disease was 70 years (range 47–88). Median Karnofsky Performance Status (KPS) was 90 (range 70–100). The most common SBRT fractionation scheme was 5 × 7 Gy (total dose 35 Gy). Response to radiotherapy was determined according to RECIST criteria v1.1. Toxicity was registered according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0. The survival analysis was performed with the Kaplan–Meier method. The correlation between time actuarial incidence and clinical parameters was studied, and the Kaplan–Meier method of log-rank test was applied.
Results: With a median follow-up of 22.68 months, local control was achieved in 89.7% of the cases. The two-year overall survival (OS) and progression-free survival (PFS) were 68% and 42%, respectively. On univariate analysis, KPS ≥80 is predictive for improved OS (p = .001) and PFS (p = .001). Acute toxicity of grade ≥2 occurred in eight (10.2%) patients and late grade ≥2 toxicity in five (6.4%) patients.
Conclusions: Ablative radiotherapy in ‘early oligometastatic state’ is a safe, effective and minimally invasive treatment modality. A good performance status (KPS ≥80) seems to influence the clinical outcome.
Introduction
The oligometastatic state identifies a subset of patients who might be amenable to curative therapy. This intermediate state of disease was first hypothesized by Hellman and Weichselbaum in 1995 and describe a cancer condition in which few metastases are present, before the tumoral cells acquire widespread metastatic potential [Citation1]. The development of modern imaging techniques, such as PET/CT, not only helped recognizing this category of patients, but also fostered a radical therapeutic approach (surgery, radiation therapy) in their treatment management [Citation2]. Before this hypothesis, the role of radiation therapy in the management of metastatic disease was restricted to palliative care only, without the ambition of interfering with disease progression. In this specific group of patients, instead, stereotactic body radiation therapy (SBRT) has been shown to reach high levels of local tumor control rates through the delivery of high doses of radiation in few fractions, without the development of significant toxicity and the morbidity and risk associated with surgical procedures [Citation3]. Any meaningful improvement in survival is debated, but SBRT could be able to delay disease progression and the need for another treatment in patients whose quality of life may be already compromised. The challenging aim is to assess whether SBRT can actually change the prognosis of those oligometastatic patients with good performance status prior to the treatment, who could mostly benefit from such radical approach. In our retrospective analysis, we analyzed the impact of SBRT on local control (LC), progression-free survival (PFS), cancer-specific survival (CSS), overall survival (OS) and toxicity in patients affected by ‘early oligometastatic disease’ characterized by a single isolated body metastasis.
Material and methods
From July 2007 to March 2016, 78 patients were treated with stereotactic body radiotherapy at our Department, for isolated body metastasis. The most frequent primary tumor was prostate cancer (28.2%), followed by colorectal cancer (23.1%), and lung cancer (20.5%). All patients received a radical treatment for the primary tumor (). More specifically, 31 (39.8%) underwent surgery, 4 (5.1%) radiotherapy and 43 (55.1%) multiple modality therapy. Median time from primary tumor treatment to SBRT for oligometastatic disease was 30.3 months (range 1.07–232.3). No patient had synchronous metastases at the time of SBRT. Median age at the diagnosis of oligometastatic disease was 70 years (range 47–88). Median Karnofsky Performance Status (KPS) was 90 (range 70–100). Patients were also evaluated by means of Charlson Comorbidity Score (CCS) [Citation4].
Table 1. Patient characteristics.
In all patients, written informed consent for radiotherapy was obtained. All patients underwent a planning CT scan in order to delineate a gross tumor volume (GTV). A planning target volume (PTV) was defined as the GTV plus a 5–10 mm isotropic margin depending on tumor location.
Organs at risk were delineated depending on the site of the GTV. The median GTV of the 78 lesions was 5.9 cc (range 0.16–110). The number of fractions was chosen considering site and volume of the metastasis. The most common fractionation scheme for the 78 lesions was 5 × 7 Gy (total dose 35 Gy). All treatment plans were normalized to ensure that 95% of the PTV received 95% of the prescribed dose. For each patient, daily image-guided radiotherapy was performed with cone beam CT, as described elsewhere [Citation5].
Patients received SBRT without concomitant systemic therapy, apart from 11/22 patients affected by hormone naïve prostate cancer, who underwent also antiandrogen deprivation therapy (ADT).
After SBRT, follow-up schedule consisted of clinical, biochemical and radiological evaluation every 3 months during the first 2 years and then every 6 months. Response to radiotherapy was determined according to RECIST criteria v1.1 (Kingston, Canada) [Citation6]. More specifically, local control was defined as disappearance of the target lesion, or as a decrease of at least 30% in the sum of the diameters of the target lesion. Toxicity was prospectively registered according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0 (Rockville, MD). Primary endpoint of this retrospective study was local control (LC), secondary endpoints were progression-free survival (PFS) defined as any radiological progression (in-field and/or out-field), overall survival (OS) and cancer-specific survival (CSS) defined as death from disease. All endpoints were measured from the end date of SBRT to the event date or to last follow-up. Finally, acute toxicity (within 90 days from the start of radiotherapy) and late toxicity (>90 days from the start of radiotherapy) were analyzed. Statistical analysis was carried out using a commercial statistical software package (SPSS 22®; SPSS Inc, Chicago, IL).
The survival analysis was performed with the Kaplan–Meier method. The correlation between time actuarial incidence and clinical parameters was studied. For all continuous parameters except for KPS, the median values were considered as cutoff levels and patients were categorized into two groups: above and equal or below the cutoff. For KPS, the value of 80 was considered appropriate. The Kaplan–Meier method of log-rank test was applied; p < .05 was considered significant. Multivariate analysis was performed using the Cox proportional-hazard regression.
Results
All patients completed the prescribed radiation treatment, with no interruption. reports treatment characteristics. The most frequent treatment site was the lung, followed by lymph nodes. Median follow-up was 22.68 months (range 1.9–95.73). At last follow-up, local control was achieved in 70 (89.7%) patients, and there were eight in-field local failures (5 lung and 3 lymph node metastases) with a one and two-year LC of 91% and 89% (), respectively (median local control was not reached). Thirty-one patients (39.7%) were progression free. Eight patients had an in-field recurrence (median time of in-field recurrence: 2.16 months, range 1.1–12.9), and 39 patients had an out-field progression (median time of out-field progression: 8 months, range 0.7–70.3). More specifically, 23/47 patients developed a widespread disease: 5 of them judged unfit for chemotherapy had supportive care, 15 were treated with chemotherapy and 3 with multiple modality treatment. The remaining 24/47 patients had an oligometastatic progression (2 of them were treated with radiotherapy, 11 with systemic therapy and 6 with multiple modality treatment). One- and two-year PFS were 57% and 42% (), respectively (median PFS was 18.8 months, 95% CI: 9.5–27.3). Forty-six (59%) patients were alive at the time of analysis and 29 (37.2%) were dead of disease. One- and two-year OS were 82% and 68% (), respectively, (median OS was 54 months, 95% CI: 26.4–73.5). One- and two-year CSS were 85% and 71%, respectively, (median CSS was 66.3 months, 95% CI: 28.7–73.5). Finally, we performed survival analysis for the main patient subgroups (prostate, colorectal and lung cancer) and for the main metastatic sites (lung and lymph nodes). Regarding prostate cancer patients, median LC and median OS were not reached, median PFS was 27.3 months (95% CI: 20.8–70.3). Lung cancer patients had a median PFS of 28.3 months (95% CI: 8–28.3), whereas median LC and median OS were not reached. Colorectal cancer patients had a median PFS of 15.17 months (95% CI: 6–28.7), a median OS of 26.1 months (95% CI: 14–66.4); median LC was not reached.
Figure 1. Kaplan–Meier curves. (a) Local control. (b) Progression-free survival. (c) Overall survival.
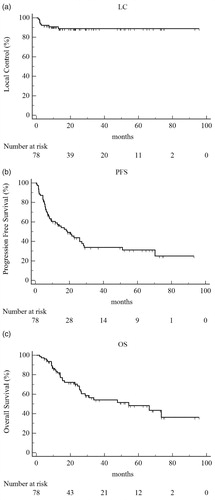
Table 2. Treatment characteristics.
Patients who received SBRT for lung metastases had a median PFS of 9.4 months (95% CI: 5.7–21.4) and a median OS of 24.1 months (95% CI: 13.9–73.5); median LC was not reached. In patients treated for lymph node metastases, median PFS was 27.3 months (95% CI: 20.8–70.3), median LC and median OS were not reached.
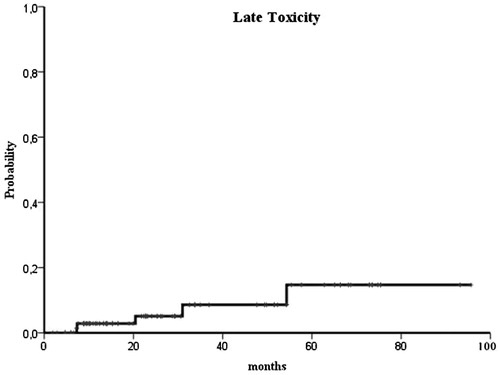
Acute toxicity of grade ≥2 occurred in eight (10.2%) patients and late grade ≥2 toxicity in five (6.4%) patients. More specifically, we registered no acute G3 toxicity, and only one patient (1.2%) developed a late G3 toxicity. Two-year late grade ≥2 toxicity was 5% ().
On univariate analysis (), KPS ≥80 represents a predictive factor associated with prolonged OS (p = .001) and PFS (p = .001). Prostate cancer histology correlates with better OS (p = .006). Cox proportional hazard-regression model showed that KPS >80 is strongly correlated with OS (p < .05, HR 8.2, CI 3.5–19.9).
Table 3. Univariate analysis.
Discussion
In our retrospective series, with a median follow-up of 22.68 months, local control was achieved in 89.7% of the cases, as in the series by Aitken et al. [Citation7]. Two-year OS and PFS were 68% and 42%, respectively. Other studies in literature [Citation8–11] report two-year survival rates in the range from 30% to 64%. We explain our finding of such a high OS value considering the clinical features of the study population. The majority of the patients (51.3%) included in our analysis were affected by the so-called favorable tumors (22 cases of prostate cancer, 18 cases of colorectal cancer) and they had a single metastasis at the time of SBRT. Eventually, 58 (74.3%) patients had a disease free interval from the radical primary tumor treatment to SBRT >12 months, and no one had synchronous oligometastases. All these good prognostic factors were identified by other authors. In the series by de Vin et al. patients with synchronous oligometastases at diagnosis had a worse OS [Citation12], and the long disease-free interval from the primary tumor treatment to the diagnosis of metastasis is considered as a good prognostic factor [Citation13]. In the series by Rusthoven et al. patients treated with SBRT for liver metastases from favorable primary tumors (colorectal and breast cancer) experienced better survival [Citation14].
In literature, the subset of oligorecurrent patients from a variety of primary sites exhibiting a less aggressive biology seems to be amenable of local therapy (surgery or ablative radiotherapy), which improves overall survival, disease progression-free survival and delay the need for systemic therapy [Citation15–20]. SBRT is very attractive because it is safe, effective and minimally invasive, leading to local control rates (from 70% to 90%) comparable with surgery [Citation19]. As proof of that, a recent international survey [Citation21] shows that clinical oncologists are increasingly using SBRT for oligorecurrent cancer despite the weak level of evidence, which is mainly based on retrospective single-institution or pooled experiences. The great interest in this treatment option has led to randomized trials such as the SABR-COMET trial. This study will compare SBRT with standard of care, in terms of overall survival and quality of life, in patients with no more than five metastatic lesions [Citation22].
However, not all oligometastatic patients seem to benefit from local therapy in terms of overall survival and progression-free survival [Citation16,Citation17], and the discrimination of patients that will have oligoprogression from those who will develop a polimetastatic state could help to decide the best therapeutic approach. In addition to clinical prognostic models, novel biomarkers such as microRNA seem to be promising in detecting the ‘true oligometastatic state’ [Citation23]. From the clinical point of view, selection criteria for oligometastatic patients candidate with SBRT are still controversial. In 2006, Kavanagh and Timmerman in their crucial paper [Citation24] identified ‘patients capable of self-care with controlled primary tumors’ as those who could mostly benefit from this specific treatment. According to Scorsetti et al. [Citation25], a KPS >70 characterizes the best candidates to be treated with SBRT for liver metastases. The role of KPS as a prognostic factor has been also investigated by other authors. In some specific sites of metastatic disease, scoring systems have been developed to select candidates for radiation therapy and to provide patients' prognosis [Citation26]. Also, Kress et al. in their work [Citation27] analyzed liver lesions treated with SBRT; at the univariate analysis, they found out that KPS >90 approached statistical significance (p = .06) for OS. Thus, they also developed their own scoring system (including KPS, number of lesions and active systemic disease) which turned out to be predictive for OS. A multinational report on SBRT for oligometastases focusing on patient selection was conducted by Dagan et al., and recently published. Most centers only considered patients for SBRT if they were free from serious comorbidities and there was a general consensus in considering a minimum PS for patient selection, even if the threshold value for the recruitment could vary between different institutions [Citation28]. In a study by Fleckenstein [Citation29] concerning oligometastatic NSCLC, KPS >90 (vs KPS 70/80) was one of the three factors that had a significant impact on PFS at univariate analysis, and it remained significant on a following multivariable Cox regression analysis. In our series, KPS ≥80 is predictive for improved OS and PFS, adding to the few reports in literature on this issue.
In conclusion, ablative radiotherapy in ‘early oligometastatic state’ represents a safe, effective and minimally invasive treatment modality. A good performance status (KPS ≥80) seems to influence the clinical outcome in this setting of patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10.
- Oh D, Ahn YC, Seo JM, et al. Potentially curative stereotactic body radiation therapy (SBRT) for single or oligometastasis to the lung. Acta Oncol. 2012;51:596–602.
- Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28–e37.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Ponti E, Ingrosso G, Carosi A, et al. Salvage stereotactic body radiotherapy for patients with prostate cancer with isolated lymph node metastasis: a single-center experience. Clin Genitourin Cancer. 2015;13:e279–e284.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)). Eur J Cancer. 2009;45:228–247.
- Aitken K, Tree A, Thomas K, et al. Initial UK experience of stereotactic body radiotherapy for extracranial oligometastases: can we change the therapeutic paradigm? Clin Oncol (R Coll Radiol). 2015;27:411–419.
- Kang JK, Kim MS, Kim JH, et al. Oligometastases confined one organ from colorectal cancer treated by SBRT. Clin Exp Metastasis. 2010;27:273–278.
- Salama JK, Hasselle MD, Chmura SJ, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118:2962–2970.
- Bae SH, Kim MS, Cho CK, et al. High dose stereotactic body radiotherapy using three fractions for colorectal oligometastases. J Surg Oncol. 2012;106:138–143.
- Milano MT, Zhang H, Metcalfe SH. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat. 2009;115:601–608.
- de Vin T, Engels B, Gevaert G, et al. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Ann Oncol. 2014;25:467–471.
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318.
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. JCO. 2009;27:1572–1578.
- Staren ED, Salerno C, Rongione A, et al. Pulmonary resection for metastatic breast cancer. Arch Surg. 1992;127:1282–1284.
- Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946.
- Tomlinson T, Robinson LA, Shell MJ, et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. JCO. 2008;26:1142–1147.
- Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830.
- Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. JCO. 2013;31:1384–1390.
- Ingrosso G, Trippa F, Maranzano E, et al. Stereotactic body radiotherapy in oligometastatic prostate cancer patients with isolated lymph nodes involvement: a two-institution experience. World J Urol. 2017;35:45–49.
- Lewis SL, Porceddu S, Nakamura N, et al. Definitive stereotactic body radiotherapy (SBRT) for extracranial oligometastases: an international survey of >1000 radiation oncologists. Am J Clin Oncol. 2015. DOI:10.1097/COC.0000000000000169
- Palma DA, Haasbeek CJA, Rodrigues GB, et al. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (SABR-COMET): Study protocol for a randomized phase II trial. BMC Cancer. 2012;12:305.
- Lussier YA, Xing HR, Salama JK, et al. MicroRNA expression characterizes oligometastasis(es). PLos ONE. 2011;6:e28650.
- Kavanagh BD, McGarry RC, Timmerman RD. Extracranial radiosurgery (stereotactic body radiotherapy) for oligometastases. Semin Radiat Oncol. 2006;16:77–84.
- Scorsetti M, Clerici E, Comito T. Stereotactic body radiation therapy for liver metastases. J Gastrointest Oncol. 2014;5:190–197.
- Agboola O, Benoit B, Cross P, et al. Prognostic factors derived from recursive partition analysis (RPA) of Radiation Therapy Oncology Group (RTOG) brain metastases trials applied to surgically resected and irradiated brain metastatic cases. Int J Radiat Oncol Biol Phys. 1998;42:155–159.
- Kress MA, Collins BT, Collins SP, et al. Scoring system predictive of survival for patients undergoing stereotactic body radiation therapy for liver tumors. Radiat Oncol. 2012;7:148.
- Dagan R, Lo SS, Redmond KJ, et al. A multi-national report on stereotactic body radiotherapy for oligometastases: patient selection and follow-up. Acta Oncol. 2016;55:633–637.
- Fleckenstein J, Petroff A, Schafers HJ, et al. Long-term outcomes in radically treated synchronous vs. metachronous oligometastatic non-small-cell lung cancer. BMC Cancer. 2016;16:348.