Abstract
Introduction: Vascular disrupting agents (VDAs) damage tumor vasculature and enhance tumor radiation response. In this pre-clinical study, we combined radiation with the leading VDA in clinical development, combretastatin A-4 phosphate (CA4P), and compared the effects seen in tumors and relevant normal tissues.
Material and methods: Radiation was applied locally to tissues in CDF1 mice to produce full radiation dose–response curves. CA4P (250 mg/kg) was intraperitoneally (i.p.) injected within 30 minutes after irradiating. Response of 200 mm3 foot implanted C3H mammary carcinomas was assessed using percent tumor control at 90 days. Normal tissue effects were evaluated using early responding skin (development of moist desquamation in the foot at 11–30 days), and late responding bladder (50% reduction in reservoir function estimated by cystometry up to 9 months after treatment), and lung (20% increase in ventilation rate measured by plethysmography within 9 months). A Chi-squared test was used for statistical comparisons (significance level of p < .05).
Results: The radiation dose controlling 50% of irradiated tumors was 52 Gy. This significantly decreased to 45 Gy with CA4P. The radiation doses inducing a change in skin, bladder and lung response in 50% of mice were 31 Gy, 14 Gy and 12 Gy, respectively. CA4P had no significant effect on the radiation response of any of these normal tissues.
Conclusions: VDAs significantly enhance tumor radiation response, but had absolutely no effect on the radiation response of early or late responding normal tissues.
Introduction
A functional tumor blood supply, formed from the host vasculature by the process of angiogenesis, is essential for meeting the oxygen and nutrient demands of the growing tumor mass [Citation1,Citation2]. Without this, tumor growth and development are inhibited. This significance has led to the development of various vascular targeting agents (VTAs), which either inhibit the angiogenesis process (angiogenesis inhibitors; AIs) or damage the already established tumor neo-vasculature (vascular disrupting agents, VDAs) [Citation3,Citation4]. These VTAs have undergone extensive pre-clinical evaluation [Citation4,Citation5] and many of them are currently in clinical development [Citation6]. However, despite them having significant inhibitory effects on tumor growth none appear capable of totally inhibiting growth or causing tumor control. Thus, their clinical potential lies in combination with more conventional therapies.
One of the more popular conventional therapies investigated in combination with VTAs is radiation [Citation4,Citation7]. This is especially true for VDAs. The rationale for this is primarily that VDAs induce extensive central necrosis, but leave a viable rim since this area contains the normal tissue vessels from which the tumor vessels develop and such normal vessels are generally unresponsive to VDA attack [Citation4,Citation5]. Since this rim region of the tumor receives its oxygen and nutrient supply from the host vessels, it is less likely to contain regions of hypoxia and thus the cancer cells are probably more radiation sensitive. Pre-clinical studies have now shown that VDAs and radiation induce tumor responses that are greater than either treatment alone, although the combined effect may not be greater than additive, and this has been observed for both single and fractionated schedules [Citation4].
Even though the combination of VDAs and radiation may be no greater than additive, this would still be of benefit provided there are no similar enhancements in normal tissue responses. Surprisingly, this is an area where there is a clear lack of data; an earlier review of published pre-clinical studies in which VDAs and radiation were combined reported some 24 studies in tumors using eight different VDAs, but only three studies investigated normal tissue reactions and that was for early responding skin damage [Citation4]. Clearly, additional normal tissue studies investigating the effects of VDAs on radiation response, especially using late responding normal tissues, should be undertaken. As a result, we now report on a study in which we compare the effects of the leading VDA in clinical development and radiation in a tumor model with what happens in both early responding skin and late responding bladder and lung.
Material and methods
Animal and tumor model
Experiments were performed using male and female CDF1 mice. The tumor model was a C3H mammary carcinoma; details of the derivation and maintenance of this model have been described previously [Citation8]. Experimental tumors were produced following sterile dissection of large flank tumors. Macroscopically viable tumor tissue was minced with a pair of scissors and 5–10 μl of this material injected into the right rear foot of 10–14 week-old mice. Experiments were performed when tumors had reached approximately 200 mm3 in size, which typically occurred 3 weeks after inoculation. Tumor volume was calculated from the formula D1 × D2 × D3 × π/6, where the D values represent the three orthogonal diameters. All animal studies were conducted according to the animal welfare policy of Aarhus University (http://dyrefaciliteter.au.dk), and with the Danish Animal Experiments Inspectorate’s approval.
Drug preparation
Combretastatin A-4 phosphate (CA4P) was kindly supplied by Mateon Pharmaceuticals (South San Francisco, CA, USA). It was freshly dissolved in sterile saline (0.9% NaCl) immediately before each experiment and intraperitoneally (i.p.) injected into mice at a constant injection volume of 0.02 ml/g mouse body weight. Drug injections were carried-out within 30 minutes following irradiation.
Tumor irradiation and response
Single dose tumor irradiations were given using a conventional therapeutic X-ray machine (240 kV, 10 mA, dose rate of 2.3 Gy/min) as previously described [Citation9]. The radiation dose was determined using an integrating chamber. All irradiations to the tumor-bearing feet were given locally to the tumors of non-anesthetized mice, which were restrained in specially constructed Lucite jigs; the tumor-bearing legs were exposed and loosely attached to the jig with tape, without impairing the blood supply to the foot. In order to irradiate only the tumors, the remainder of the mouse was shielded by 1 cm of lead. To secure homogeneity of the radiation dose, the tumors were immersed in a water bath set at 25 °C with about 5 cm of water between the X-ray source and the tumor. Following irradiation, the animals were returned to their cages, observed on a weekly basis, and the percentage of animals in each treatment group showing local tumor control at 90 days, determined.
Skin damage
Normal skin response to radiation was achieved by locally irradiating the right rear foot of restrained, non-anesthetized mice, in a similar fashion as explained for the tumor studies; full details of the normal skin irradiations have been described previously [Citation9,Citation10]. However, to maintain the leg in the correct position for treatment, and still avoid using anesthetics, a small drop of histoacrylic glue was applied to the restraining jig in the region of the uppermost part of the leg. Using tape the leg was then compressed against the jig for 5-minutes, following which the tape was loosened and left for a further 10-minutes prior to treatment. After treatment, the leg was easily separated from the jig by clipping the hair. Mice were returned to their cages and observed daily from 11 to 30 days after treatment. A scoring system had previously been developed for defining different levels of damage in mouse foot skin [Citation10] and for our current study we used a reversible moist desquamation level affecting 75% of the skin area; the percentage of animals in each treatment group developing this level of damage was recorded.
Bladder damage
Bladder damage following radiation was estimated by measuring bladder reservoir function as previously described [Citation11,Citation12]. Anesthetized animals (pentobarbital anesthesia injected i.p. at 60 mg/kg), were transferred to a cylindrical jig that could be positioned inside a lead box designed so that only the lower abdomen was irradiated (dose rate 2.9 Gy/minute). Repeated cystometry was performed before and up to 9 months following irradiation. To achieve this, female mice were anesthetized and the bladder emptied by insertion of a catheter through the urethra. This catheter was then replaced by another fluid filled cannula connected via a catheter to a home-built infusion pump. Via a three-way valve the system was connected to a pressure transducer and an ink-jet recorder. The bladder was then gradually filled with isotonic saline at room temperature using an infusion rate of 0.1 ml/minute. This infusion was stopped when leakage occurred around the catheter. The endpoint, established previously [Citation11], was a 50% reduction in bladder volume compared to the pretreatment value at an intravesicular pressure of 20 mmHg; the volume at this pressure being obtained from each cystometry curve.
Lung damage
Radiation-induced lung damage was assessed by measuring lung ventilation rate as described previously [Citation12,Citation13]. This was achieved by restraining non-anesthetized mice in specially constructed jigs and then transferring these jigs to a lead holder that allowed for local lung irradiation only (dose rate 1.9 Gy/minute). Measurements of mouse ventilation rate were made before and bi-weekly up to 9 months after irradiating, by placing the animals in a home-built whole body plethysmograph. Those animals showing a ventilation rate that was 20% higher than the pretreatment value, were recorded; that endpoint had previously been established [Citation12].
Data and statistical analysis
The endpoints selected for the tumor and all normal tissues studies have been shown to be reproducible for radiation dose–response studies [Citation9–13]. Thus, for each tissue full radiation dose–response curves were produced for either radiation alone or radiation and CA4P. From these curves, logit analysis allowed us to calculate the radiation dose necessary to induce a response in 50% of animals, with 95% confidence intervals. The ratio of these values obtained for radiation alone and radiation with CA4P were used to calculate enhancement ratios, which were then compared using a Chi-squared test; the significance level being p < .05.
Results
Logit analysis of the radiation dose–response curve obtained when irradiating C3H mammary carcinomas () resulted in a value of 52 Gy for the radiation dose necessary to control 50% of irradiated tumors (). Similar analysis of the radiation dose–response curve obtained when mice were also treated with CA4P () significantly decreased the radiation dose controlling 50% of mice to 45 Gy. Comparing these radiation doses resulted in an enhancement ratio of around 1.2 (). Similar dose–response relationships were obtained for the development of moist desquamation in the mouse foot skin for radiation alone and radiation with CA4P (). However, unlike the results found with tumors, injecting CA4P after irradiating had no significant effect on the development of moist desquamation; the radiation dose inducing moist desquamation in 50% of mice was 31 Gy for radiation alone and 32 Gy for radiation with CA4P ().
Figure 1. The effect of CA4P (250 mg/kg) on the radiation response of tumors and normal skin. Full radiation dose–response curves were produced and show the percentage of animals with local tumor control at 90 days after treating a C3H mammary carcinoma (left panel) or developing moist desquamation between days 11 and 30 in normal foot skin (right panel). For both tumor and skin, the symbols are for radiation alone (^) or radiation + CA4P (•), based on an average of 14 mice/group for tumor and six mice/group for skin. Lines through the data were fitted following logit analysis.
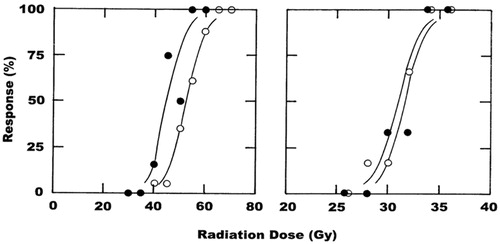
Table 1. Summary effects of radiation and CA4P in tumors and normal tissues.
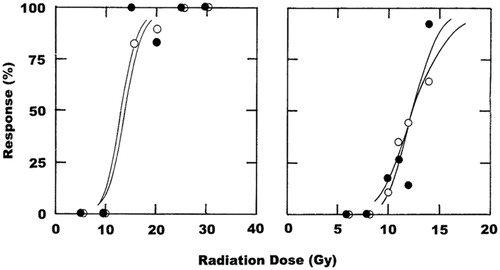
The radiation dose–response curves obtained when mouse bladder and lung were irradiated revealed almost identical effects with or without CA4P treatment (). For bladder, the radiation dose that induced a 50% reduction in bladder volume in 50% of irradiated mice was calculated to be 14 Gy for radiation alone and non-significantly reduced to 13 Gy when radiation and CA4P were combined (). In lung, the radiation dose causing a 20% increase in ventilation rate in 50% of treated animals was determined to be around 12 Gy regardless of whether mice were treated with CA4P or not ().
Figure 2. The effect of CA4P (250 mg/kg) on the radiation response of normal bladder and lung. Full radiation dose–response curves were produced and show the percentage of animals that up to 9 months after treatment have either a 50% reduction in bladder reservoir function estimated by cystometry (left panel), or a 20% increase in lung ventilation rate measured by plethysmography (right panel). For both bladder and lung, the symbols are for radiation alone (^) or radiation + CA4P (•), based on an average of seven mice/group for bladder and 16 mice/group for lung. Lines through the data were fitted following logit analysis.
Discussion
Our study clearly showed that irradiating the C3H mammary carcinoma and then injecting mice with CA4P significantly enhanced radiation response. An enhanced response is entirely consistent with that previously reported for CA4P in this tumor model and other rodent models [Citation4], and with CA4P analogs [Citation4,Citation14]. The growth inhibitory effect of CA4P [Citation15] and the drugs ability to enhance radiation response [Citation16] have been shown to be drug dose-dependent, with 250 mg/kg giving the maximum effect in this C3H mammary carcinoma, although in other tumor models significant enhancements have been seen with much lower doses [Citation4]. This effect is also schedule dependent, in that although a benefit was seen when CA4P is administered after irradiating, no enhancement occurs when CA4P is administered prior to irradiation [Citation15,Citation17]. The reduction in tumor perfusion induced by CA4P results in substantial cell killing and necrosis induction [Citation15]. Those cells most likely to die first are those hypoxic tumor cells that are already under reduced oxygen and nutrient conditions. Studies with invasive oxygen electrodes [Citation18–20], noninvasive positron emission tomography [Citation20,Citation21], and even radiation response [Citation18,Citation19] not only confirm that our C3H mammary carcinoma model contains significant regions of hypoxia, but that it accounts for some 10–20% of the total viable population [Citation18,Citation19]. These hypoxic cells are those resistant to radiation, and although they survive following irradiation, they are then likely killed by the subsequent CA4P treatment. Previous reports of a lack of enhanced radiation response when CA4P was administered prior to irradiation [Citation15,Citation17], despite CA4P itself killing a significant proportion of tumor cells, suggests that a population of normally radiation sensitive cells were made hypoxic by the drug treatment and survive, thus becoming a source of resistance to any subsequent radiation treatment. Administering CA4P after irradiating, as in the current study, avoids this issue.
Since CA4P and radiation target different cell populations, it suggests that the optimal combination of these treatments will only result in an additive response. This would be a therapeutic benefit provided the similar effects were not found in relevant normal tissues. Our results clearly show that using the optimal sequence of CA4P and radiation that improves tumor response has no significant influence on the radiation response of both early responding skin and late responding bladder and lung normal tissues. CA4P induces tubulin depolymerization in cells and this ultimately leads to cell death [Citation22]. Although this can affect any cell type it appears that with short exposure times, as likely to be seen in vivo due to the relatively rapid drug clearance [Citation22], dividing endothelial cells are particularly sensitive [Citation23]. One would primarily expect to find dividing endothelial cells associated with developing tumor vasculature and not in normal tissues, thus a tumor specific effect, as we found, is most likely. Previous estimates of blood perfusion clearly showed a substantial reduction after CA4P treatment in this C3H mammary carcinoma model with the same high dose as used in the current study, but found absolutely no effect in skin perfusion [Citation24]. Surprisingly, there were significant improvements in perfusion in both bladder and lung, but since neither of these tissues is hypoxic any increase in perfusion and corresponding change in oxygen delivery to these tissues would not be expected to influence radiation response.
In our study, we only used single dose treatments and there remains the question of what would happen when using more clinically relevant fractionation schedules. Administering CA4P in a limited fractionated schedule (10 fractions in 12 days) was investigated in this C3H mammary carcinoma model, again using the same high drug concentration [Citation17]. CA4P not only enhanced the fractionated treatment, it also resulted in an enhancement ratio of 1.10, which was similar to the value of 1.16 found in our current study. Our lack of any normal tissue enhancement using large single doses is unlikely to influence radiation response of normal tissues in a fractionated schedule, although confirmation might be necessary.
Acknowledgments
The author thanks Ms. Dorthe Grand and Ms. Inger Marie Horsman for excellent technical help with the experiments and animal care.
Disclosure statement
The author reports no conflicts of interest.
Additional information
Funding
References
- Brem S, Brem H, Folkman J, et al. Prolonged tumor dormancy by prevention of neovascularization in the vitreous. Cancer Res. 1976;36:2807–2812.
- Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture Cancer Res. 1986;46:467–473.
- Siemann DW, Bibby MC, Dark G, et al. Differentiation and definition of vascular-targeted therapies. Clin Cancer Res. 2005;11:416–420.
- Horsman MR, Siemann DW. Pathophysiological effects of vascular targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006;66:11520–11539.
- Siemann DW, Horsman MR. Modulation of the tumor vasculature and oxygenation to improve therapy. Pharmacol Ther. 2015;153:107–124.
- National Cancer Institute Web Site. Available from: http://www.cancer.gov/clinicaltrials
- Siemann DW, Warrington KH, Horsman MR. Targeting tumor blood vessels: an adjuvant strategy for radiation therapy. Radiother Oncol. 2000;57:5–12.
- Overgaard J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Int J Radiat Oncol Biol Phys. 1980;6:1507–1515.
- Horsman MR, Chaplin DJ, Overgaard J. Combination of nicotinamide and hyperthermia to eliminate radioresistant chronically and acutely hypoxic tumor cells. Cancer Res. 1990;50:7430–7436.
- von der Maase H. Effect of cancer chemotherapeutic drugs on the radiation-induced skin reactions in mouse feet. Br J Radiol. 1984;57:697–707.
- Lundbeck F, Ulsø N, Overgaard J. Cystometric evaluation of early and late irradiation damage to the mouse urinary bladder. Radiother Oncol. 1989;15:383–392.
- Horsman MR, Siemann DW, Chaplin DJ, et al. Nicotinamide as a radiosensitizer in tumours and normal tissues: the importance of drug dose and timing. Radiother Oncol. 1997;45:167–174.
- von der Maase H, Overgaard J, Vaeth M. Effect of cancer chemotherapeutic drugs on the radiation-induced lung damage in mice. Radiother Oncol. 1986;5:245–257.
- Wittenborn TR, Horsman MR. Targeting tumour hypoxia to improve outcome of stereotactic radiotherapy. Acta Oncol. 2015;54:1385–1392.
- Murata R, Siemann DW, Overgaard J, et al. Interaction between combretastatin A-4 disodium phosphate and radiation in murine tumors. Radiother Oncol. 2001;60:155–161.
- Nielsen T, Murata R, Maxwell RJ, et al. Preclinical studies to predict efficacy of vascular changes induced by combretastatin a-4 disodium phosphate in patients. Int J Radiat Oncol Biol Phys. 2008;70:859–866.
- Iversen AB, Busk M, Horsman MR. Induction of hypoxia by vascular disrupting agents and the significance for their combination with radiation therapy. Acta Oncol. 2013;52:1320–1326.
- Horsman MR, Khalil AA, Nordsmark M, et al. Relationship between radiobiological hypoxia and direct estimates of tumour oxygenation in a mouse tumour model. Radiother Oncol. 1993;28:69–71.
- Horsman MR, Khalil AA, Siemann DW, et al. Relationship between radiobiological hypoxia in tumors and electrode measurements of tumor oxygenation. Int J Radiat Oncol Biol Phys. 1994;29:439–442.
- Bentzen L, Keiding S, Horsman MR, et al. Assessment of hypoxia in experimental mice tumours by [18F]fluoromisonidazole PET and pO2 electrode measurements: influence of tumour volumes and carbogen breathing. Acta Oncol. 2002;41:304–312.
- Bentzen L, Keiding S, Horsman MR, et al. Feasibility of detecting hypoxia in experimental mouse tumours with 18F-fluorinated tracers and positron emission tomography: a study evaluating [18F]fluoromisonidazole and [18F]fluoro-2-deoxy-d-glucose. Acta Oncol. 2000;39:629–637.
- Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435.
- Dark GD, Hill SA, Prise VE, et al. Combretastatin A-4, an agent that displays potent and selective toxicity towards tumor vasculature. Cancer Res. 1997;57:1829–1834.
- Murata R, Overgaard J, Horsman MR. Comparative effects of combretastatin A-4 disodium phosphate and 5,6-dimethylxanthenone-4-acetic acid on blood perfusion in a murine tumour and normal tissues. Int J Radiat Biol. 2001;77:195–204.
