Abstract
Background: Dose-guided adaptive radiation therapy (DGART) is the systematic evaluation and adaptation of the dose delivery during treatment for an individual patient. The aim of this study is to define quantitative action levels for DGART by evaluating changes in 3D dose metrics in breast cancer and correlate them with clinical expert evaluation.
Material and methods: Twenty-three breast cancer treatment plans were evaluated, that were clinically adapted based on institutional IGRT guidelines. Reasons for adaptation were variation in seroma, hematoma, edema, positioning or problems using voluntary deep inspiration breath hold. Sixteen patients received a uniform dose to the breast (clinical target volume 1; CTV1). Six patients were treated with a simultaneous integrated boost to CTV2. The original plan was copied to the CT during treatment (re-CT) or to the stitched cone-beam CT (CBCT). Clinical expert evaluation of the re-calculated dose distribution and extraction of dose-volume histogram (DVH) parameters were performed. The extreme scenarios were evaluated, assuming all treatment fractions were given to the original planning CT (pCT), re-CT or CBCT. Reported results are mean ± SD.
Results: DVH results showed a mean dose (Dmean) difference between pCT and re-CT of -0.4 ± 1.4% (CTV1) and −1.4 ± 2.1% (CTV2). The difference in V95% was −2.6 ± 4.4% (CTV1) and −9.8 ± 8.3% (CTV2). Clinical evaluation and DVH evaluation resulted in a recommended adaptation in 17/23 or 16/23 plans, respectively. Applying thresholds on the DVH parameters: Dmean CTV, V95% CTV, Dmax, mean lung dose, volume exceeding 107% (uniform dose) or 90% (SIB) of the prescribed dose enabled the identification of patients with an assumed clinically relevant dose difference, with a sensitivity of 0.89 and specificity of 1.0. Re-calculation on CBCT imaging identified the same plans for adaptation as re-CT imaging.
Conclusions: Clinical expert evaluation can be related to quantitative DVH parameters on re-CT or CBCT imaging to select patients for DGART.
Introduction
Radiotherapy to the chest wall or breast improves the outcome after mastectomy and breast conserving surgery for breast cancer [Citation1–3]. An important aspect of radiotherapy is to verify patient positioning during treatment to ensure accurate dose deposition. In general, Electronic Portal Images (EPIs) are used to verify positioning during breast irradiation, by matching anatomical structures or markers to digitally reconstructed radiographs [Citation4]. Additionally cone‐beam computed tomography (CBCT) can be integrated in clinical practice to evaluate 3D patient anatomy and positioning during treatment. Both methods can be used to select patients for adaptive radiation therapy based on image guidance (IGRT). This selection is based on pre-defined clinical action levels for changes in patient anatomy or positioning [Citation5,Citation6].
Changes in patient anatomy or positioning, however, do not directly translate into differences in dose deposition [Citation7–9], which is the main factor influencing tumor control or side-effects after radiotherapy. Dose-guided radiation therapy (DGRT) is the systematic evaluation of the dose delivery during treatment for an individual patient with the aim to adapt the treatment if needed. Preferably, adaptation is only performed if there is a dosimetric impact of the radiotherapy treatment on the target volume or organs at risk, translating into an impact on local control or toxicity. In breast radiotherapy, an important aim is to limit the dose to the heart. The heart dose can be affected by breathing motion, therefore, breath hold techniques are frequently applied when treating the left breast to limit anatomical shifts [Citation10,Citation11].
Recalculation of the dose during treatment can be performed on a new CT (re-CT) or by using the imaging information of the CBCT during treatment. For lung cancer patients the use of CBCT for treatment evaluation was already performed [Citation12]. In this study, we will investigate the ability to use a re-CT or CBCT imaging for dose guidance in breast cancer patients, with the aim to define quantitative action levels based on dose or volume measures that relate to clinical expert evaluation.
Material and methods
Patients
In 2016, three percent (N = 24) of our breast cancer plans (N = 882) were adapted, after visual assessment of the anatomy according to institutional IGRT guidelines. In short, breast cancer patients are positioned on EPI using a surgical clip match [Citation13]. Surgical clips are delineated and expanded with a margin of 2 mm. On EPI the clips should fit within the expanded clip structures or only one of the clips is allowed to have shifted within 5 mm. If this situation is correct, the position of sternum, clavicular and rib arch are evaluated, these should be within 8 mm. In addition the rotation of the patient should be within three degrees and skin contour has to be within an 8 mm margin. If these criteria are not met, the patient is re-positioned and re-evaluation takes place. If there is still a disagreement further actions are taken, i.e., the match procedure is performed on CBCT and consultation of radiotherapist oncologist and/or medical physicist is advised.
One plan was excluded from the analysis, due to an adaptation in positioning support materials for re-CT imaging and further treatment. Therefore, we reevaluated 23 treatment plans of 22 breast cancer patients. Reasons to adapt the radiotherapy treatment were changes in seroma (N = 18), hematoma (N = 1), edema (N = 1), voluntary moderately deep inspiration breath hold (VMDIB; N = 2) or positioning (N = 1). In one patient, adaptation was performed twice, due to repeated changes in seroma. Patient characteristics are shown in .
Table 1. Patient characteristics.
Radiation treatment
Treatment planning was performed using forward IMRT-planning as described by Peulen et al. [Citation14], In short, treatment planning was started with classic tangential beams and optimization was performed by adding segments to the tangential beams before adding beams at other angles to achieve a uniform dose to the breast. In case of a simultaneous integrated boost (SIB), Volumetric Arc Therapy (VMAT) was used in addition. Dose calculation was performed using the analytical anisotropic algorithm (AAA) implemented in Eclipse (version 10.0, Varian Medical systems, Palo Alto, CA, USA). Patients were treated with either a uniform dose to the clinical target volume (CTV1) of 16 fractions of 2.66 Gy (Total dose: 42.6 Gy; N = 16) or a SIB technique, irradiating the CTV1 to a total dose of 45.6 Gy in 21 fractions (N = 5) or 46.7 Gy in 23 fractions (N = 2) while simultaneously treating the CTV tumourbed (CTV2) to a total dose of respectively 55.9, 58.0 or 61.2 Gy. Adaptation was performed after 2 to 12 fractions for the patients treated with a homogeneous dose, and after 1 to 17 fractions for patients receiving a SIB technique.
CT imaging
An original planning CT (pCT) and re-CT was available for all adapted treatment plans. In eleven cases a CBCT was available within a maximum time interval of one day to the re-CT. CT images were acquired using a Siemens Somatom Sensation Open (Siemens Healthcare, Erlangen, Germany), using three mm slice thickness. CBCT imaging was performed with the Varian TrueBeam on-board imaging (Varian Medical Systems, Palo Alto, CA, USA), using the low-dose thorax CBCT protocol (half-fan bowtie filter, 3600, 125 kV, 20mA). To secure equivalency between CT and CBCT a Hounsfield Unit calibration with the Catphan® 504 phantom (The Phantom Laboratory, Salem, NY) was performed on all on-board imaging systems every six months, according to the standard Varian procedure (TrueBeam STx 1.5 Technical Reference Guide, Volume 2 – Imaging, September 2010, Varian medical systems)[Citation12].
During (CB)CT imaging, patients were positioned in supine position, on a flat tabletop using a laser alignment system with the arms above the head in an arm support. The vmDIBH technique was performed as described by Brouwers et al. [Citation15]. CBCT images were stitched to the planning CT to extend the field of view in longitudinal direction, using home-made software. Re-CT and CBCT images were rigidly registered to pCT to allow propagation of treatment plans and contours.
Contouring
For all patients, target volumes and organs at risk were contoured on both the pCT and re-CT by two experienced radiation oncologists in consensus according to the ESTRO atlas [Citation16]. After rigid registration, the contours of the re-CT were copied to the stitched CBCT. Since the CTV of the breast/chest wall was defined at five mm under skin surface for pCT and re-CT, the copied CTV on CBCT was adapted accordingly. All other contoured structures on CBCT were accepted after rigid registration.
Analysis
The original treatment plan was calculated on the pCT, re-CT and stitched CBCT to evaluate the effect of anatomical changes on the dose distribution. In addition, the adapted treatment plan was calculated on the re-CT to evaluate the optimal dose distribution available for the new anatomy.
Clinical expert evaluation of the dose distribution of the original treatment plan on the re-CT was performed by a radiation oncologist and clinical physicist to estimate the clinical relevance of the performed plan adaptation. A visual evaluation of the isodose lines was performed within the planning system (Eclipse, Varian Medical systems, Palo Alto, CA, USA). The target volume coverage (V95%) and potential over dosage in the body (V107%, V90%) were evaluated, in addition the dose to organs at risk was assessed.
In addition, DVH parameters were evaluated for the plans calculated on the pCT, re-CT and stitched CBCT images. For the CTVs, the evaluated DVH parameters were the mean dose (Dmean) and volume receiving at least 95% of the prescribed dose (V95%). Also, the mean dose to the heart (MHD) and lungs (MLD) were calculated. For patients receiving a uniform dose to the breast, the maximum dose (Dmax) in the body and the volume exceeding 107% of prescribed homogeneous dose (V107%) were defined. In case of a SIB technique, the Dmax was defined in the body structure excluding the planned boost volume (PTV2) with an additional margin of 1.5 cm (HELP-SIB volume). This HELP-SIB volume was also used to evaluate the volume exceeding 90% of the prescribed boost dose (V90%) in the elective part. The extreme scenarios were evaluated, assuming all treatment fractions were given to pCT, re-CT or CBCT.
DVH parameters for the CBCT and the re-CT were compared to investigate if the CBCT can be used as a surrogate for the re-CT imaging.
Dose distribution changes were considered significant when:
Dmean in CTV1 or CTV2 decreased with more than 2%.
V95% in CTV1 or CTV2 decreased with more than 5%
Dmax in Body or Help-SIB increased with more than 2%
V107% in Body or V90% in Help-SIB increased with more than 20 cm3
MLD increased with more than 0.5 Gy
MHD increased with more than 0.5 Gy
Finally, the relationship between clinical expert evaluation and the use of objective DVH criteria was studied.
Statistics
Reported are mean ± standard deviation and the range of the analyzed DVH parameters. A Pearson’s correlation coefficient was calculated between changes in CTV1 volume and DVH parameters. A Wilcoxon signed rank test was used to evaluate dose differences between reCT and CBCT imaging. Last, the sensitivity and specificity of DVH thresholds on re-CT or CBCT imaging were evaluated in comparison to expert evaluation.
Results
This study retrospectively analyzed the dosimetric impact of clinical changes in anatomy or positioning for 23 treatment plans of 22 breast cancer patients. PlanID 1-17 were plans with a homogeneous dose to CTV1, PlanID 18-23 included a SIB.
Clinical expert evaluation resulted in a recommended plan adaptation in 17/23 treatment plans. In one additional plan repeated imaging was advised to monitor changes. The main reason for adaptation was a decrease in coverage of the CTV1 or CTV2 in 7/17 treatment plans. In 9/17 cases, the reason for adaptation was an overdosage, mainly described as the volume exceeding 107% and in 1/17 cases an increased mean lung dose occurred, exceeding the institutional constraint for lung.
The evaluated DVH parameters for the original plan on the pCT and re-CT, including the dosimetric differences are summarized in and . An example of the evaluated dose distributions is visualized in . There was a moderate negative correlation between the CTV1 volume change and the change in Dmean CTV1 (R = −0.7) or change in CTV1 V95% (R = −0.5).
Figure 1. Box plots show the lower, upper quartile and the median line of the differences in dose metrics between the original plan on pCT and recalculation on the re-CT. The mean is indicated with ‘x’.
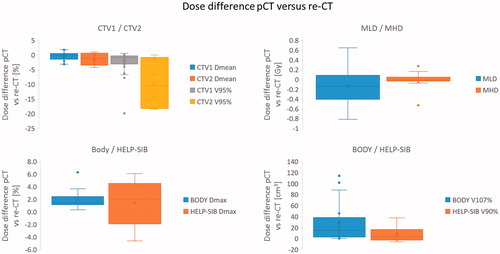
Figure 2. Example of a patient (PlanID 4) treated with a homogeneous dose to the right breast (16 x 2.66Gy) after surgical lumpectomy. Visualized are the original plan on the pCT, the adapted (new) plan on the re-CT and a re-calculation of the dose using the original plan on the re-CT, respectively. Notice the reduction in seroma on re-CT imaging, which was the reason for re-planning.
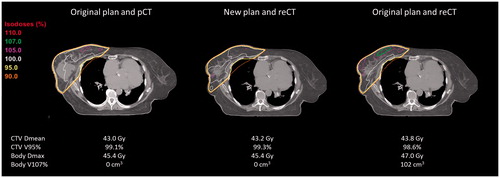
Table 2. The evaluated DVH parameters for the original plan on the pCT or re-CT, including the dosimetric differences.
In 10 out of 11 available CBCT the CTV1 was not totally within field of view in longitudinal direction. In one patient the field of view of the CBCT was too small in the transverse plane to visualize the full body contour of the patient. Nevertheless, all CBCT scans were used for evaluation.
provides an example of the re-calculation of the original plan on re-CT or CBCT imaging. The differences in DVH parameters between re-calculation on the stitched CBCT versus re-CT analysis were on average 0.4 ± 0.7% (Dmean CTV1), 1.5 ± 0.5% (Dmean CTV2), 0.9 ± 1.9 (V95% CTV1), 2.3 ± 3.3% (V95% CTV2), 0.0 ± 0.1Gy (MHD), 0.0 ± 0.1Gy (MLD), −0.1 ± 1.3% (Dmax Body), −0.2 ± 1.0% (Dmax Help-SIB), 4.2 ± 33 cm3 (V107%) and −0.5 ± 28 cm3 (V90%; Supplementary Table 1).
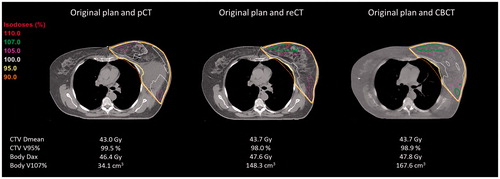
Figure 3. Example of a patient (PlanID 13) treated with a homogeneous dose to the left breast and axilla level 3 + 4 (16 x 2.66Gy) after lymph node dissection of an unknown primary lesion. Visualized are the original plan on the pCT, the re-calculation of the dose on the re-CT and stitched CBCT, respectively.
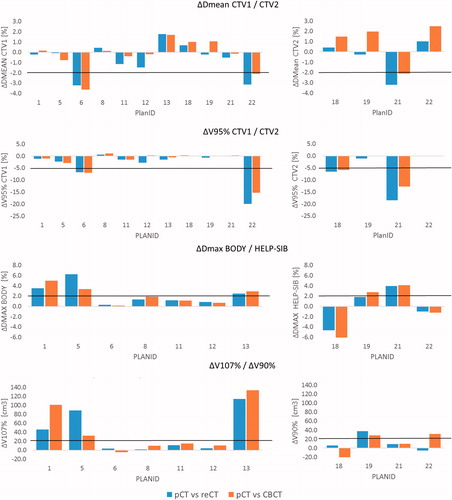
shows that only small differences in DVH parameters were observed when comparing treatment plans based on the re-CT or on the CBCT. Wilcoxon signed rank test showed that the dose difference after re-calculation on re-CT or CBCT imaging was not significantly different for all evaluated DVH parameters; ΔDmean CTV1 (p = .12), ΔDmean CTV2 (p = .07), ΔV95% CTV1 (p = .10), ΔV95% CTV2 (p = .11), ΔDmax Body (p = .87), ΔDmax Help-SIB (p = .72), ΔV107% Body (p = .55), ΔV90% Help-SIB (p = 1.0), ΔMLD (p = .32), ΔMHD (p = 1.0).
Figure 4. Visualized are the dose differences in DVH parameters. (i) Between the original plan on pCT and recalculation on the re-CT and (ii) between the original plan on pCT and re-calculation on the stitched CBCT, respectively. DVH thresholds for adaptation are marked in red. Dose differences are similar between reCT and CBCT imaging; the Wilcoxon signed rank test was non-significant for all parameters.
Evaluating the relationship between expert evaluation and DVH thresholds we observed that for:
Coverage: Using the DVH thresholds for Dmean and V95% in the CTV it was found that 7/23 plans would have required adaptation of which five included a SIB. A disagreement between clinical evaluation and selected thresholds was PlanID 20 which fails the selected thresholds (ΔDmean CTV2: −2.1%, ΔV95% CTV2: −18.2%), but was clinically recommended for follow-up. Another mismatch was observed for PlanID 12, which did not exceed the selected criteria (ΔDmean CTV1: –1.5%, ΔV95% CTV1: −2.8%) however, on clinical judgment required adaptation due to a decreased coverage.
Overdose: Using the DVH thresholds for Dmax, V107% or V90% we were able to select 8/9 cases for adaptation based on clinical judgment. One plan (PlanID 11) did not exceed the DVH criteria (ΔDmax Body: 1.2%; ΔV107 Body: 11 cm3), however was on clinical judgment selected for adaptation.
Lung dose: The DVH threshold of >0.5 Gy MLD difference was exceeded for two PlanID, of which one was also reported by clinical evaluation. The other plan was clinically already selected for adaptation based on over dosage in the body.
Overall, using the combination of all the DVH criteria 16/23 plans would require a plan adaptation. In relationship to clinical expert evaluation, assuming that the plan selected for treatment monitoring on clinical evaluation should exceed the DVH thresholds, we observed a sensitivity and specificity of 0.89 and 1.0, respectively. Applying the predefined DVH thresholds to the CBCT, would have led to recommendation of plan adaption in the same 8/11 plans, as applying the thresholds to the re-CT. Results are summarized in One can appreciate that not all plans that were clinically adapted based on IGRT guidelines provided a dosimetrically significant difference. In is shown that PlanID 2, 8, 9, 10 or 17 were in clinical practice adapted, however on both clinical expert re-evaluation of the dose distribution and using the DVH thresholds, this adaptation was not relevant.
Table 3. Overview of the clinical and DVH evaluation for adaptive treatment.
Discussion
This study was initiated to define quantitative action levels for breast cancer patients based on dosimetric information which relate to clinical expert evaluation, to be able to objectively select patients for adaptive radiotherapy. The adaptive strategy aims to improve the radiation treatment by re-optimizing the treatment plan using systematic feedback during treatment [Citation6]. It was shown in 23 breast cancer plans, selected for adaptation based on IGRT that the use of dosimetric, quantitative action levels can be used to assure systematic feedback, which is correlated to ‘subjective’ clinical expert evaluation.
Re-calculation of the 3D dose of the original plan was performed on the re-CT with anatomical change. A clinical expert evaluation of the dose distribution and a quantitative analysis of selected DVH parameters were performed. We showed that not all anatomical changes need an adaptive treatment, 17/23 plans would require adaptation based on clinical evaluation, while this was the case for 16/23 plans using the DVH thresholds. Note that, although anatomical changes appeared to be large; i.e., the clinical target volume changed with −36% to 85%, the dosimetric impact was limited. This is due to the used treatment technique, which is based on tangential fields and the used margins. The CTV1 mean dose difference ranged from −3.2 to 1.8%, assuming the worst case scenario. The additional value of DGART was already reported for lung cancer patients, showing that only half of the patients with an observed clinical change actually needed an adaptive treatment [Citation9,Citation17]. In this study we confirm that dosimetric information has also additional value to image guided radiotherapy for breast cancer patients. However, it should be realized that although dose volume parameters have a better clinical relevance than geographic set-up parameters, a next step should be to estimate the effect of the difference in DVH parameters on local control or on normal tissue complications, to determine the actual clinical relevance [Citation18].
The incidence of adaptation is highly dependent on the defined dosimetric criteria. The chosen DVH thresholds provide a good agreement between clinical expert and DVH evaluation. The same decision was proposed for 21 out of 23 treatment plans. However, using the threshold DVH values, patients will be more objectively selected for DGART. In addition, it provides opportunities to automate the decision process. In a next step, we could use 2D or 3D portal dosimetry to detect the assumed clinical relevant dose differences. Previously Nijsten et al. [Citation19] showed for 20 breast cancer patients that changes in the mean PTV dose larger than 5% and changes in V95% larger than 10% could be accurately predicted by a 2D portal dosimetry method combined with 2D gamma functions. The current results show that clinically one would like to detect smaller differences in DVH parameters so probably a 3D method is needed for this.
Remarkably, the anatomical changes had only a minor influence on MHD and MLD which, in the worst case, increased with 0.3 Gy and 0.6 Gy, respectively. The observed difference in MHD was acceptable and in the same range as observed during a course of breath hold breast cancer radiotherapy, which was <0.5Gy as reported by Dunkerley et al. [Citation20]. In the current study, the increased MLD was the decisive factor for adaptation in one plan only.
The results of this study are dependent on the treatment technique. Our results show that the use of two tangential fields including segments to treat the breast/chest wall (CTV1) is quite robust to clinical changes. However, the boost volume (CTV2) was treated with VMAT, with a CTV to PTV margin of 0.5cm and therefore more sensitive to changes in anatomy or positioning. Note that all target volumes were re-delineated by radiation oncologists on re-CT imaging. It is known from previous studies that target delineation of the boost volume is highly affected by inter-observer variability [Citation21]. This inter-observer variability in the target volume delineation on pCT and re-CT could have affected the evaluated dose parameters.
In clinical practice re-CT imaging is not standard available. Therefore, the use of CBCT imaging as a surrogate for re-CT imaging was evaluated. Note that the usability of CBCT scans for dosimetry will depend on Hounsfield Unit calibration, CBCT image quality and delineation accuracy [Citation12,Citation22]. We observed average differences between recalculation on re-CT and CBCT imaging <1% for CTV1 Dmean, V95% and Dmax in the Body or HELP-SIB. Larger differences were observed for the boost volume, nevertheless also for CTV2 the difference for Dmean was <2% and for V95% <3%. These results are in the same range as the dose differences previously reported in lung cancer patients [Citation12]. Most importantly, this accuracy was proven sufficient to select breast cancer patients for adaptation on CBCT imaging using the previously described DVH criteria.
There are some limitations of this study. First, we performed a rigid registration of the re-CT or CBCT to the pCT. Using this method we analyzed the most optimal match, omitting variable changes in patient positioning. In clinical practice the EPI are used to match patient positioning. A previous study of Topolnjak et al. [Citation23] showed that setup uncertainties are larger using EPID position verification in comparison to CBCT. On the other hand, Batumalai et al. [Citation24] showed recently that there is in general no significant difference between EPI and CBCT setup. Second, we used a subjective measurement as a gold standard for adaptation, namely, the clinical interpretation of a radiation oncologist and clinical physicist in consensus. However, this is current clinical practice. The ability to relate this subjective decision to objective DVH thresholds provides the possibility to attach guidelines for DGART. Third, the patient group selected for this study was very heterogeneous i.e. regarding target volume, prescribed dose and breath hold technique. Nevertheless, since our dosimetric thresholds were defined as a difference between pCT and re-CT, instead of absolute dose values, its seems reasonable to apply the same thresholds for the whole population. Last, the field of view (FOV) of the CBCT images was limited. Due to relative large target volumes the CTV was not always fully present in the FOV of the CBCT. By stitching the CBCT to the pCT assumptions are made regarding the dose distribution. However, as results show, this method still enables to detect reliably patients sensitive to clinically relevant dose changes.
To conclude, not all IGRT observed changes provide a clinically significant variation in the evaluated DVH parameters. Dosimetric differences are larger for the CTV receiving a SIB. It is possible to relate clinical expert evaluation to quantitative DVH parameters on re-CT or CBCT imaging. This can be used to systematically and more accurately select patients for DGART.
IONC_A_1349334_Supplementary_Information.docx
Download MS Word (14.6 KB)Disclosure statement
MAASTRO has research agreements with Varian Medical Systems.
References
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106.
- Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716.
- McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135.
- Salm A, Strijbos J, Dijcks C, et al. Use of skin markers and electronic portal imaging to improve verification of tangential breast irradiation. Radiother Oncol. 2009;90:106–109.
- Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012;9:688–699.
- Yan D, Vicini F, Wong J, et al. Adaptive radiation therapy. Phys Med Biol. 1997;42:123–132.
- Wolfs C, Gomes Bras M, Schyns L, et al. Detection of anatomical changes in lung cancer patients with 2D time-integrated, 2D time-resolved and 3D time-integrated portal dosimetry: a simulation study. Phys Med Biol. Forthcoming. [cited 2017 Jun 5]. DOI:10.1088/1361-6560/aa7730
- Schmidt ML, Hoffmann L, Kandi M, et al. Dosimetric impact of respiratory motion, interfraction baseline shifts, and anatomical changes in radiotherapy of non-small cell lung cancer. Acta Oncol. 2013;52:1490–1496.
- Moller DS, Khalil AA, Knap MM, et al. Adaptive radiotherapy of lung cancer patients with pleural effusion or atelectasis. Radiother Oncol. 2014;110:517–522.
- Koivumaki T, Tujunen J, Viren T, et al. Geometrical uncertainty of heart position in deep-inspiration breath-hold radiotherapy of left-sided breast cancer patients. Acta Oncol. 2017;56:879–883.
- Verhoeven K, Sweldens C, Petillion S, et al. Breathing adapted radiation therapy in comparison with prone position to reduce the doses to the heart, left anterior descending coronary artery, and contralateral breast in whole breast radiation therapy. Pract Radiat Oncol. 2014;4:123–129.
- de Smet M, Schuring D, Nijsten S, et al. Accuracy of dose calculations on kV cone beam CT images of lung cancer patients. Med Phys. 2016;43:5934.
- van der Salm A, Murrer L, Steenbakkers I, et al. Actual target coverage after setup verification using surgical clips compared to external skin markers in postoperative breast cancer radiotherapy. Pract Radiat Oncol. Forthcoming. [cited 2017 Apr 19]. DOI:10.1016/j.prro.2017.04.012
- Peulen H, Hanbeukers B, Boersma L, et al. Forward intensity-modulated radiotherapy planning in breast cancer to improve dose homogeneity: feasibility of class solutions. Int J Radiat Oncol Biol Phys. 2012;82:394–400.
- Brouwers PJ, Lustberg T, Borger JH, et al. Set-up verification and 2-dimensional electronic portal imaging device dosimetry during breath hold compared with free breathing in breast cancer radiation therapy. Pract Radiat Oncol. 2015;5:e135–e141.
- Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3–10.
- Persoon LC, Egelmeer AG, Ollers MC, et al. First clinical results of adaptive radiotherapy based on 3D portal dosimetry for lung cancer patients with atelectasis treated with volumetric-modulated arc therapy (VMAT). Acta Oncol. 2013;52:1484–1489.
- Stick LB, Yu J, Maraldo MV, et al. Joint estimation of cardiac toxicity and recurrence risks after comprehensive nodal photon versus proton therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2017;97:754–761.
- Nijsten SM, van Elmpt WJ, Mijnheer BJ, et al. Prediction of DVH parameter changes due to setup errors for breast cancer treatment based on 2D portal dosimetry. Med Phys. 2009;36:83–94.
- Dunkerley N, Bartlett FR, Kirby AM, et al. Mean heart dose variation over a course of breath-holding breast cancer radiotherapy. Br J Radiol. 2016;89:20160536.
- Verhoeven K, Peeters S, Erven K, et al. Boost delineation in breast radiation therapy: isotropic versus anisotropic margin expansion. Pract Radiat Oncol. 2016;6:e243–e248.
- Zhang H, Tan W, Sonke JJ. Effect of compressed sensing reconstruction on target and organ delineation in cone-beam CT of head-and-neck and breast cancer patients. Radiother Oncol. 2014;112:413–417.
- Topolnjak R, Sonke JJ, Nijkamp J, et al. Breast patient setup error assessment: comparison of electronic portal image devices and cone-beam computed tomography matching results. Int J Radiat Oncol Biol Phys. 2010;78:1235–1243.
- Batumalai V, Phan P, Choong C, et al. Comparison of setup accuracy of three different image assessment methods for tangential breast radiotherapy. J Med Radiat Sci. 2016;63:224–231.
