Abstract
Introduction: More than 50% of patients with locally advanced cervical cancer (LACC) have pathological nodes. Coverage probability (CovP) is a new planning technique allowing for relaxed dose at the boost periphery minimising collateral irradiation. The aim was to report the first early clinical outcome data for CovP based simultaneous integrated boost (SIB) in LACC.
Material and methods: Twenty-three consecutive node positive patients were analysed. FIGO stage IB2/IIB/IIIB/IVA/IVB was 1/14/3/1/4. Treatment was radio(chemo)therapy (RT) delivering 45 Gy/25 fx whole pelvis ± para-aortic region (PAN) using volumetric arc therapy (VMAT) followed by magnetic resonance imaging (MRI) guided brachytherapy. PAN RT (13 pts) was given if >2 nodes or if node(s) were present at the common iliac vessels or PAN. Nodal gross tumour volumes (GTV-N) were contoured on both PET-CT and MRI. Clinical target volume (CTV-N) was formed by fusion of GTV-NCT and GTV-NMRI. A 5-mm isotropic margin was used for planning target volume (PTV-N). Nodes in the small pelvis were boosted to 55.0 Gy/25 fx. Common iliac and para-aortic nodes received 57.5 Gy/25 fx. Planning aims for CovP were PTV-N D98 ≥ 90%, CTV-N D98 ≥ 100% and CTV-N D50 ≥ 101.5%.
Results: Seventy-four nodes were boosted. A consistent 5.0 ± 0.7 Gy dose reduction from CTV-N D98 to PTV-N D98 was obtained. In total, 73/74 nodes were in complete remission at 3 months PET-CT and MRI. Pelvic control was obtained in 21/23 patients. One patient (IB2, clear cell) had salvageable local disease, while another (IIB) failed in a boosted node. Two patients failed in un-irradiated PAN. One patient age 88 (IIIB) did not receive PAN RT, despite a common iliac node. The other (IIB) recurred above L1. Two further patients (IVB) failed systemically.
Conclusion: Since complete remission at 3 months is predictive for favourable long-term nodal control, our study indicates that CovP for SIB is promising.
Introduction
For patients with locally advanced cervical cancer (LACC) the increasing use of image guided adaptive brachytherapy (IGABT) has provided a very high rate of local control (90% to 95%) with an accompanying improvement of overall survival which likely amounts to 10% [Citation1–7]. Alongside, the risks of severe gastro-intestinal, urological and vaginal morbidities have decreased by about 50% compared to point-A based brachytherapy (BT) [Citation2,Citation3]. Since the introduction of concomitant chemotherapy almost 20 years ago [Citation8], there has unfortunately been limited improvement of the outcome of definitive radio-chemotherapy by use of new chemotherapeutic drugs and/or biological modifiers. Currently, the results of a randomised study on adjuvant chemotherapy with carboplatin and paclitaxel are awaited to see if adjuvant treatment with conventional drugs improves survival [Citation9].
As local control is now very high and in view of the limited progress within medical oncology, regional nodal control becomes increasingly important as possible target for further improvements of the therapeutic index by securing adequate doses of radiation to an adequate target volume [Citation10]. Thus, pelvic control remains a problem even in the face of the success of IGABT with 3-year nodal failure in 11% [Citation11] and 5-year pelvic failure rates in stage IIB–IIIB reported to be in the range 13–33% [Citation6]. In parallel to IGABT, new advanced external beam radiotherapy (EBRT) techniques for imaging, contouring, treatment planning and treatment delivery has become available for LACC which provides new avenues for delivering discrete doses to nodal targets adapted to the likelihood of micro- and macro-metastatic regional disease [Citation12,Citation13], which is expected also to reduce EBRT induced morbidity.
During the last decade a multicentre group around the GEC-ESTRO (Groupe Européen de Curiethérapie and European SocieTy for Radiotherapy and Oncology) gynaecological network and the EMBRACE study (an intErnational study on MRi guided BRachytherapy in LACC) has grown [Citation14]. Through this cooperation a new clinical protocol – EMBRACE II, was launched in the spring of 2017 (www.embracestudy.dk). A main focus in EMBRACE II is optimisation of EBRT in terms of dose to involved nodes, dose to elective regional stations and a risk adapted strategy for extended radiation to the para-aortic region (PAN). As a part of the development of the EMBRACE II study, it has previously been shown that an equivalent dose of 60 Gy in 2 Gy fx (EQD2) including the dose contribution from BT provides a high rate of control of pathological nodes [Citation12,Citation15]. Furthermore, it is possible to use coverage probability treatment planning (CovP) to provide a narrow margin simultaneous integrated boost (SIB) [Citation12]. In fact, with CovP it is feasible to both increase the SIB dose centrally in the pathological node and decrease dose at the edge of the nodal planning target volume (PTV-N), thereby reducing incurring high dose volumes into adjacent organs at risk (OAR) [Citation12]. The theoretical framework for CovP dose planning has previously been described [Citation16,Citation17]. In short, CovP supplements probability information about geometric uncertainties to the classic PTV concept to allow for a relaxed planning aim at the edge of PTV.
Since CovP is a new planning technique in LACC, it is the aim of this study to report the first dosimetric and early clinical outcome data for CovP based SIB in patients with LACC in the context of a comprehensive treatment description according to the planning aims and dose constraints of the EMBRACE II protocol.
Material and methods
Patients
Staging included magnetic resonance imaging (MRI), (18)F-flourodeoxy-glucose positron emission tomography-computed tomography (FDG PET-CT) and gynaecological examination in general anaesthesia performed collaboratively by an oncologist and a gynaecologist. Clinical decision regarding treatment was based on a multidisciplinary conference including specialists in pathology, oncology, gynaecology, radiology and nuclear medicine. The diagnosis was confirmed by biopsies from the uterine cervix in all cases. Nodes were considered pathological according to the EMBRACE II criteria: FDG-PET positive, short axis ≥1 cm on CT or MRI and/or short axis between 0.5 and 1.0 cm on MRI with pathological morphology (irregular border, high signal intensity and/or round shape). Histological confirmation of the nodes either by fine needle aspiration or laparoscopic staging was not performed. In total, 23 consecutive patients with 74 pathological nodes initiating definitive radio(chemo)therapy including CovP planned SIB between November 2015 and November 2016 were included in the analysis. Patients with para-aortic nodes beyond L1 and patient with distant metastases were not included and referred for palliative treatment.
Median age of the patients was 57 years () with FIGO IIB as the dominant stage (61%). Most patients had squamous cell carcinoma (87%). The number of pathological nodes per patient varied from 1 to 10 with 13/23 (57%) of the patients having 1 to 2 nodes and 10/23 (43%) having 3 or more nodes. Fifty-one of the 74 pathological nodes (69%) were found in the small pelvis and 17/74 (23%) at the common iliac vessels and 6/74 (8%) in the PAN.
Table 1. Patient characteristics of 23 node positive patients with locally advanced cervical cancer treated with definitive radio(chemo)therapy employing coverage probability for planning of simultaneous integrated boosts of the pathological nodes.
Contouring and treatment planning for EBRT
All patients were scanned employing both MRI and PET-CT with full bladder in the supine treatment position. A bladder filling protocol was enforced. A CT scan with empty bladder was also obtained and all scans were co-registered for contouring in the Eclipse system (Varian Medical Systems, Palo Alto, CA, USA).
Contouring was initiated on MRI focusing on the tumour related gross tumour volumes (GTV) and clinical target volumes (CTV) according to the EMBRACE II protocol (GTV-T, CTV-THR-initial, CTV-TLR). PET was used for identification of pathological nodes, which then were contoured individually (GTV-Nn) on MRI and CT and fused to form CTV-Nn. The elective target (CTV-E) including the regional nodal stations was contoured on CT with support from MRI. In all patients, every pelvic nodal station to the level of the aortic bifurcation was included in CTV-E. In one patient with involvement of the distal 1/3 of the vagina, the groins were also included in CTV-E. Apart from an elderly patient age 88 years, PAN to the level of the renal vessels was included systematically if more than two pathological nodes were found or if node(s) were present at the common iliac vessels or higher. In total, PAN was included in 13/23 (57%) of the patients (). Based on the information from the fused images including full and empty bladder scans an internal target volume (ITV45) was constructed including CTV-E and CTV-Nn with in principle zero margin and an individualised margin around the central tumour related target (CTV-T). The contours were reviewed at a radiology conference before dose planning. An isotropic margin of 5 mm was used around ITV45 and around each CTV-Nn to form PTV45 and PTV-Nn, respectively. Dose planning using volumetric arch therapy (VMAT) was performed using the planning aims and dose volume histogram (DVH) constraints of EMBRACE II. The planning aim for PTV45 was V42.75 Gy >95%. Volume of body outer contour treated to minimally 43 Gy (body V43) was used for evaluating the conformity of the elective part of the EBRT plans by calculating the conformity index = body V43/PTV45 volume.
Table 2. Planning aim and prescribed dose volume histogram parameters for 23 node positive patients with locally advanced cervical cancer treated with VMAT according to the EMBRACE II protocol.
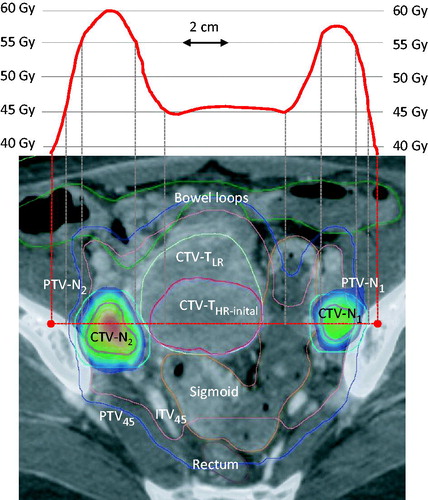
Based on previous analysis on the dose–response following SIB of pathological nodes [Citation15] and the expected dose contribution from BT [Citation18] the planning aim was to deliver 55 Gy/25 fx to nodes in the small pelvis and 57.5 Gy/25 fractions to nodes further away. In total, 47 pathological nodes in the small pelvis were boosted to 55.0 Gy/25 fx. Twenty-three nodes at the common iliac vessels or PAN received 57.5 Gy/25 fx. A further three nodes placed presacrally or just below the bifurcation of the common iliac vessels also received 57.5 Gy. A single very large node (CTV-N volume 83 cm3) close to an external iliac vessel was planned for 60 Gy due to its size. SIB boosting of nodes with CovP techniques is described in detail in Ramlov et al. [Citation12]. A dose gradient between CTV-N and PTV-N was constructed during dose planning to reflect less frequent occupancy of lymph nodes in the periphery of the PTV as compared to the central part. According to Ramlov et al., the planning aims PTV-N D98 ≥ 90%, CTV-N D98 ≥ 100% and CTV-N D50 ≥ 101.5% of prescribed dose facilitate dose gradients which reflect typical uncertainties in image guided cervix cancer radiotherapy and which secure the dose delivery to be sufficiently robust to inter-fractional variations [Citation12]. An example of dose plan and pelvic dose profile for SIB of two pathological nodes behind the external iliac vessels using CovP is demonstrated in .
Figure 1. Coverage probability based treatment plan for simultaneous integrated boost of two pathological nodes behind the external iliac vessels (CTV-N1 and CTV-N2) with 55 Gy/25 fx combined with whole pelvic and para-aortic 45 Gy/25 fx using VMAT in a patient with stage IVB disease. The patient had five additional nodes (N3–N7) at higher levels all boosted to 57.5 Gy (not shown). The dose profile across the pelvis is shown in the upper panel. The tumour related targets GTV-Nn, GTV-T (not shown), CTV-THR-initial and CTV-TLR were contoured on MRI and merged with CT (lover panel). In this case, GTV-T was identical to CTV-THR-initial as the whole cervix was tumour infiltrated. CTV-Nn was obtained by fusing GTV-NMRI and GTV-NCT. ITV45 was contoured taking into account the possible movement of the GTV-T, CTV-THR-initial and CTV-TLR as judged from target movements observed between MRI, CT full and CT empty bladder. The margin for PTV-Nn and PTV45 was 5 mm. The rectum, sigmoid and outer contour of bowel and other relevant organs (not shown on this slide) were contoured on CT. Notice the homogenous 45 Gy dose plateau in the central pelvis where brachytherapy was later applied. The colour wash represents the range 52.3 Gy (95% of 55 Gy) to 59.8 Gy (Dmax of CTVN2).
Concomitant chemotherapy
Concomitant chemotherapy was delivered as weekly Cisplatin during EBRT at a dose of 40 mg/m2 according to the randomised study by Rose et al. [Citation19]. Chemotherapy was preferably delivered on a Monday or Tuesday but was paused/discontinued if leucocytes were <2.5 × 109/l, thrombocytes <50 × 109/l or glomerular filtration rate <50 ml/min. Concomitant chemotherapy was also not administered if the completion of EBRT and IGABT was considered in danger due to age, comorbidity and/or poor general condition. Weekly blood tests and chrome-EDTA clearance performed before and after three courses of cisplatin were used routinely to guide the administration of concomitant chemotherapy.
Image guided adaptive brachytherapy
All patients received two fractions of pulsed dose rate BT at the end of an intended 50 days maximal overall treatment time (OTT) including BT. The implantation technique, imaging, contouring, and treatment planning of image guided adaptive BT at Aarhus University Hospital has previously been described [Citation2,Citation20,Citation21]. The planning aim and DVH constraints according EMBRACE II which was in force for planning of BT in these patients has been given in .
Table 3. EMBRACE II planning aim and prescribed external beam radiotherapy, concomitant chemotherapy and image guided adaptive brachytherapy for 23-node positive patients with locally advanced cervical cancer.
Follow-up
All patients were examined with PET-CT and MRI at 3 months after treatment using the RECIST criteria to categorize the response. About 1 week later, gynaecological examination in general anaesthesia was performed and relevant biopsies were taken if uncertainties were found on imaging. Routinely, MRI was planned for 12 months follow-up. Additional scans were done as per symptoms. Patient charts were reviewed end of April 2017 for local, regional, systemic and vital status. Follow-up time was calculated from initiation of radiotherapy. Groups were compared using χ2-test or t-test as appropriate. A significance level of 5% was used. A probability <.05 (two-sided) was considered to indicate significance.
Results
The mean volume of body V43 () was 1363 and 1959 cm3 for patients treated without PAN and with PAN, respectively. The constraint for the conformity index <1.10 improved over time as 5 of the first 11 patients versus 12 of the last 12 patients fulfilled this criterion (p < .001). For bowel, rectum, bladder and sigmoid, the soft constraints for V30Gy and V40Gy were fulfilled in at least 80% of the patients except for rectum and bladder V40Gy which were fulfilled in 8/13 (62%) of PAN patients. EBRT and BT were completed within an OTT ≤50 days in 20/23 (87%) of the patients (). Only one patient had an OTT >55 days. The CTVHR-initial decreased by a factor of about three from 110 cm3 (≈diameter 6 cm) to 37 cm3 (≈diameter 4 cm) for CTVHR at BT (p = .010). The intracavitary/interstitial (IC/IS) implant approach was overall used in 16/23 (70%) of patients, increasing from 57% to 89% depending on CTVHR volume being below or above 30 cm3, respectively. The EQD2 hard constraints on total EBRT + BT dose of the EMBRACE II protocol were fulfilled in all patients except one patient marginally surpassing 75 Gy D2cm3 for rectum. The median number of cycles of concomitant chemotherapy was 4 and 10/23 received all five planned cycles. The major reasons for less than five cycles were: leucopenia (four pts), insufficient kidney function (three pts), poor performance status (three pts) or age/patient refusal (three pts).
The DVH parameters obtained for the 74 boosted nodes are given in . The planning aims for CovP of SIB were accomplished for CTV-N D50, CTV-N D98 and PTV-N D98 in 73/74 (99%), 71/74 (96%) and 73/74 (99%) of the nodes, respectively. In the five instances of under dosage, this was marginal varying between 0.1 and 0.4 Gy. As demonstrated in (left panel) there was no obvious relationship between CTV-N D98 and the boost volume. The same observation was found for the CovP dose gradient and we observed a consistent 5.0 ± 0.7 Gy dose reduction from CTV-N D98 to PTV-N D98 (right panel). Thus, the dose gradient over the 5 mm margin from the edge of CTV-N to PTV-N was 1 Gy/mm. The dose reduction was increased to 6.4 ± 0.9 Gy from the central part of the node (CTV-N D50) to PTV-N D98. There was a small increase in dose towards the nodal centre as seen by the 1.3 Gy difference (p < .001) between CTV-N D98 and CTV-N D50.
Figure 2. Left panel: Log-linear plot of CTV-N D98 as a function of CTV-N volume obtained by coverage probability planned simultaneous integrated boost of 74 nodes in 23 patients with locally advanced cervical cancer. Right panel: The dose reductions obtained from the central part of the node (CTV-N D50) and periphery of the node (CTV-N D98) to the periphery of the nodal PTV (PTV-N D98) as a function CTV-N volume.
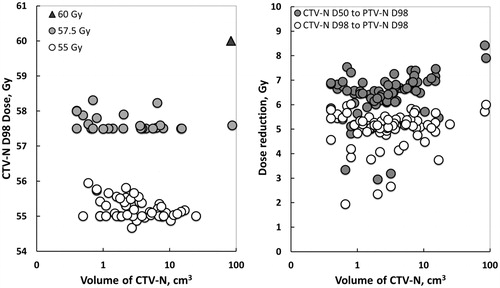
Table 4. Dose volume parameters for coverage probability planned simultaneous integrated boost of 74 individual nodes in 23 patients with locally advanced cervical cancer treated according to the EMBRACE II protocol.
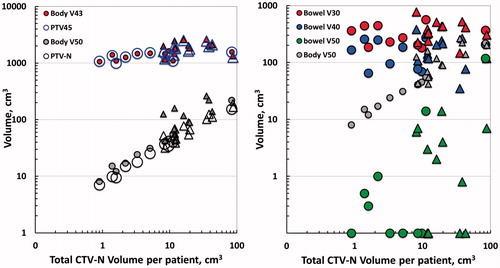
The impact of the total boosted volume (total CTV-N volume per patient) for irradiation of OAR was further investigated in log–log plots (). The boost volume did not influence the conformity index (V43/PTV45) as the increase in V43 was exactly paralleled by the increasing PTV45 (left panel). Increased PTV45 volume with increased CTV-N volume was caused by an increasing number of patients receiving PAN irradiation with increased number of nodes (triangles). A steeper relationship was found for V50/PTV-N. The influence of the boosted volume towards irradiation of bowel showed no influence at 30 and 40 Gy, whereas the volume of bowel treated to 50 Gy increased steeply albeit displaced by a factor of about 10 compared to V50Gy within in the entire body outline. For bladder, sigmoid and rectum, there was no evident relationship between boosted volume (total CTV-N volume) and V30Gy, V40Gy and V50Gy (data not shown).
Figure 3. Left panel: Target volume (PTV45, PTV-N) and volume of outer contour of body treated to 43 and 50 Gy (Body V43, Body V50) as a function total boost volume per patient (total CTV-N volume) in 23 patients with locally advanced cervical cancer treated with coverage probability planned simultaneous integrated boost. Right panel: Treated volume of bowel at three dose levels (Bowel V30, V40 and V50Gy) as a function of total CTV-N volume. Body V50 is plotted for comparison. Zero values of Bowel V50Gy were set to 0.1 cm3. Patients treated with PAN are indicated by triangles (both panels).
In total, 73/74 (99%) of the boosted nodes were in complete remission at 3 months on PET-CT and MRI. With a median follow-up time of 9 months a total of 22/23 (96%) of the patients were alive and 19/23 patients (83%) had disease control. Local and pelvic control with radio(chemo)therapy and IGABT was so far 22/23 (96%) and 21/23 (91%), respectively. One patient (IB2, clear cell adenocarcinoma) had persistent but surgically salvageable local disease with regional/systemic control. Another patient (IIB) obtained local control but failed in a boosted 1.1 cm3 node in the small pelvis boosted with 55 Gy/25 fx. The patient also developed a new opposite parametrical node inside ITV45. On review of the contouring, DVH parameters, daily cone beam CT (CBCT) scans obtained during EBRT as well as the BT dose contribution we found no apparent explanation for the nodal failure in this patient. Two patients failed in un-irradiated PAN. One was the patient age 88 (IIIB) that did not receive PAN RT due to age despite a common iliac node. The patient succeeded in obtaining complete remission following salvage RT of PAN including CovP boost of the recurrent nodes. The other patient with PAN recurrence (IIB) recurred above L1. Two further patients (both stage IVB) failed systemically but obtained local and regional remission including the PAN region.
Discussion
To our knowledge, this is the first report on dosimetric and early clinical outcome from CovP based treatment planning of SIB in LACC. At 3 months, 73/74 of the boosted nodes were in complete radiological and metabolically remission. Although this is very early clinical results, previous data indicate that complete nodal remission at first follow-up after definitive radio(chemo)therapy is a strong predictor for favourable long-term outcome [Citation13]. Thus, with a median follow-up time of 29 months, Vargo et al. demonstrated an excellent 3-year regional control rate of 94% with only 1/179 involved nodes progressing after IMRT based SIB. The SIB dose was similar to our study 55 Gy/25 fx. Including the biological effect of accelerated radiotherapy [Citation13] and the dose contribution from BT [Citation12], the present dose levels for CovP based SIB seem appropriate for securing a biologically effective dose >60 Gy EQD2 for all nodes [Citation12,Citation15]. The added value of a high local control rate [Citation1,Citation2,Citation5] preventing re-seeding [Citation10] and the possibility for an abscopal effect [Citation22–24] points to a possible impact of such a high rate of regional control on improvement of disease free survival and overall survival.
We observed that CovP provided a consistent physical dose reduction of 5–6 Gy towards the surrounding OAR in both small and large nodes independent of anatomical position and intended dose level for the SIB. Including the effect of dose per fraction, the dose reduction enlarges to 7–7.5 Gy EQD2 (α/β = 3) across the 5-mm CTV-N to PTV-N margin, thereby minimising the disadvantage of applying SIB with dose per fraction >2 Gy in regions of PTV-N overlap with OAR. The CovP dose gradient is also of increasing importance during the course of EBRT as the nodes regress leaving space for OAR [Citation12]. Re-planning after 2–3 weeks may therefore be considered in case of nodes with very large volumes, where regression is anticipated.
As previously discussed, a reduction of normal tissue high dose volumes of the magnitude obtained by CovP is expected to be of clinical importance for morbidity reduction, even when used in combination with a narrow PTV-N margin [Citation12]. The average V50 values of 50–100 cm3 (maximum of 263 cm3) observed in the present study in patients with multiple nodes should be evaluated in the context of the practice in many institutions still to administer whole pelvic 50.4 Gy EBRT with an expected V50 of 1500–2500 cm3. This is a further argument to lower the elective dose from 50 to 45 Gy with focal CovP planned SIB for pathological nodes as used in EMBRACE II [Citation25].
However, the high dose volume reduction is just one element in the context of EMBRACE II aiming for a general normal tissue sparing also from reducing the volume treated to moderate doses (i.e. V43). This is expected to be obtained by delivering IMRT/VMAT based on MRI guided contouring of anatomically defined individual target elements (GTV-T, CTV-THR-initial, CTV-TLR, CTV-E), personalised margins for internal motion and tight PTV margins as used in the present group of patients. Based on the preliminary experience presented here the ambitious EMBRACE II goals for the dose volume parameters both for EBRT and for EBRT and BT combined seem within reach even with an unfavourable group of patients with positive nodes ( and ). A learning curve was observed as the conformity index was improved over time. Regular audit of the DVH parameters is therefore recommended and is taking place for centres participating in EMBRACE II.
Despite the favourable DVH parameters obtained, we failed to deliver concomitant chemotherapy to the extend foreseen in EMBRACE II as less than half of the patients received five cycles of cisplatin (). The reasons for this were in 9/13 (69%) cases not directly related to EBRT (e.g. age, poor performance status, reduced kidney function) likely reflecting that our cohort was consecutive and unselected with regard to the EMBRACE II criteria. For the four cases of leucopenia, PAN RT was delivered in two, but in all cases the conformity index was ≤1.10 and the V43 within the EMBRACE II limits in 3/4. The use of CovP for SIB or the addition of dose volume constraints for bone marrow will therefore likely not improve the general ability for delivering concomitant chemotherapy.
The FIGO staging system is still used for cervical cancer although nodal status is not included [Citation26]. Surgical staging of pelvic and para-aortic nodes by laparoscopic intervention can be used to obtain certain knowledge on the regional nodes [Citation27]. However, surgical staging and nodal debulking has no proven therapeutic effect in itself [Citation28]. On the contrary, survival may be compromised [Citation29,Citation30] and complications such as infection, lymphocele, lymphoedema and port-metastases are potential risks that may not only postpone but also severely curb the effect of definitive radio-chemotherapy, which including IGABT is the treatment of choice in LACC [Citation30]. With the inclusion of PET-CT and MRI for target delineation and dose planning new possibilities are available for identification and efficient eradication of regional pathological nodes.
Without histological proof there is a risk for treating false positive nodes with excess doses of radiation [Citation31]. However, with CovP-based SIB even large nodal volumes can be addressed without significant dose spillage into the surroundings as shown in the present study. About 25% of patients with PET positive pelvic nodes have false PET negative scans for nodes in the PAN [Citation32], implying a higher risk for para-aortic failures in particular for these patients. One approach to this problem is to deliver prophylactic PAN RT to all patients with PET CT positive pelvic nodes, which seems feasible in terms of regional control and severe G3–4 morbidity [Citation13]. Knowing that the volume treated to intermediate dose levels (e.g. body V43) is of importance for moderate G2 morbidity, an alternative is to deploy a risk adapted strategy such as used in EMBRACE II, where PAN RT is delivered to all patients > two nodes or nodes at the common iliac vessels or PAN. A third and not mutually exclusive alternative to EMBRACE II is to use minimal invasive techniques such as laparoscopic inframesenteric para-aortic lymph-node dissection to tailor the use of PAN RT as currently being tested in the LILACS study [Citation33].
In conclusion, this report on early clinical outcome following definitive radio(chemo)therapy and IGABT in LACC points to CovP as a selective and safe approach for treatment planning of a SIB of pathological nodes in patients with LACC. The planning aims of the recently launched EMBRACE II protocol with regard to both EBRT and IGABT seem obtainable. Long-term oncological outcome including the performance of the risk-adapted strategy for PAN RT is expected to be available in about 400 node positive patients when the EMBRACE II study is completed.
Disclosure statement
The authors reported no potential conflict of interest.
Additional information
Funding
References
- Pötter R, Georg P, Dimopoulos JC, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–123.
- Lindegaard JC, Fokdal LU, Nielsen SK, et al. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol. 2013;52:1510–1519.
- Rijkmans EC, Nout RA, Rutten IH, et al. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol. 2014;135:231–238.
- Sadozye AH, Reed N. A Review of recent developments in image-guided radiation therapy in cervix cancer. Curr Oncol Rep. 2012;14:519–526.
- Mazeron R, Castelnau-Marchand P, Dumas I, et al. Impact of treatment time and dose escalation on local control in locally advanced cervical cancer treated by chemoradiation and image-guided pulsed-dose rate adaptive brachytherapy. Radiother Oncol. 2015;114:257–263.
- Sturdza A, Pötter R, Fokdal LU, et al. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120:428–433.
- Tanderup K, Eifel PJ, Yashar CM, et al. Curative radiation therapy for locally advanced cervical cancer: brachytherapy is NOT optional. Int J Radiat Oncol Biol Phys. 2014;88:537–539.
- Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–786.
- Thomas G. Are we making progress in curing advanced cervical cancer? J Clin Oncol. 2011;29:1654–1656.
- Beadle BM, Jhingran A, Yom SS, et al. Patterns of regional recurrence after definitive radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2010;76:1396–1403.
- Nomden C, de Leeuw AAC, Tanderup K, et al. Nodal failure after chemoradiation and magnetic resonance imaging guided adaptive BT in cervical cancer: a subanalysis within embrace. Int J Radiat Oncol Biol Phys. 2016;96:S12.
- Ramlov A, Assenholt MS, Jensen MF, et al. Clinical implementation of coverage probability planning for nodal boosting in locally advanced cervical cancer. Radiother Oncol. 2017;123:158–163.
- Vargo JA, Kim H, Choi S, et al. Extended field intensity modulated radiation therapy with concomitant boost for lymph node-positive cervical cancer: analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int J Radiat Oncol Biol Phys. 2014;90:1091–1098.
- Tanderup K, Lindegaard JC, Kirisits C, et al. Image guided adaptive brachytherapy in cervix cancer: A new paradigm changing clinical practice and outcome. Radiother Oncol. 2016;120:365–369.
- Ramlov A, Kroon PS, Jurgenliemk-Schulz IM, et al. Impact of radiation dose and standardized uptake value of (18)FDG PET on nodal control in locally advanced cervical cancer. Acta Oncol. 2015;54:1567–1573.
- Baum C, Alber M, Birkner M, et al. Robust treatment planning for intensity modulated radiotherapy of prostate cancer based on coverage probabilities. Radiother Oncol. 2006;78:27–35.
- Stroom JC, de Boer HC, Huizenga H, et al. Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability. Int J Radiat Oncol Biol Phys. 1999;43:905–919.
- Mohamed SM, Aagaard T, Fokdal LU, et al. Assessment of radiation doses to the para-aortic, pelvic, and inguinal lymph nodes delivered by image-guided adaptive brachytherapy in locally advanced cervical cancer. Brachytherapy. 2015;14:56–61.
- Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153.
- Fokdal L, Tanderup K, Hokland SB, et al. Clinical feasibility of combined intracavitary/interstitial brachytherapy in locally advanced cervical cancer employing MRI with a tandem/ring applicator in situ and virtual preplanning of the interstitial component. Radiother Oncol. 2013;107:63–68.
- Tanderup K, Nielsen SK, Nyvang G, et al. From point A to the sculpted pear: MR image guidance significantly improves tumour dose and sparing of organs at risk in brachytherapy of cervical cancer. Radiother Oncol. 2010;94:173–180.
- Desar IM, Braam PM, Kaal SE, et al. Abscopal effect of radiotherapy in a patient with metastatic diffuse-type giant cell tumor. Acta Oncol. 2016;55:1510–1512.
- Takaya M, Niibe Y, Tsunoda S, et al. Abscopal effect of radiation on toruliform para-aortic lymph node metastases of advanced uterine cervical carcinoma – a case report. Anticancer Res. 2007;27:499–503.
- Levy A, Chargari C, Marabelle A, et al. Can immunostimulatory agents enhance the abscopal effect of radiotherapy? Eur J Cancer. 2016;62:36–45.
- Mazeron R, Petit C, Rivin E, et al. 45 or 50 Gy, which is the optimal radiotherapy pelvic dose in locally advanced cervical cancer in the perspective of reaching magnetic resonance image-guided adaptive brachytherapy planning aims? Clin Oncol (R Coll Radiol). 2016;28:171–177.
- Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104.
- Marnitz S, Kohler C, Roth C, et al. Is there a benefit of pretreatment laparoscopic transperitoneal surgical staging in patients with advanced cervical cancer? Gynecol Oncol. 2005;99:536–544.
- Cheung TH, Lo KW, Yim SF, et al. Debulking metastatic pelvic nodes before radiotherapy in cervical cancer patients: a long-term follow-up result. Int J Clin Oncol. 2011;16:546–552.
- Brockbank E, Kokka F, Bryant A, et al. Pre-treatment surgical para-aortic lymph node assessment in locally advanced cervical cancer. Cochrane Database Syst Rev. 2013;3:CD008217.
- ICRU report 89. Prescribing, recording, and reporting brachytherapy for cancer of the cervix. Bethesda (MD): J.ICRU; 2013.
- Loft A, Berthelsen AK, Roed H, et al. The diagnostic value of PET/CT scanning in patients with cervical cancer: a prospective study. Gynecol Oncol. 2007;106:29–34.
- Gouy S, Morice P, Narducci F, et al. Prospective multicenter study evaluating the survival of patients with locally advanced cervical cancer undergoing laparoscopic para-aortic lymphadenectomy before chemoradiotherapy in the era of positron emission tomography imaging. J Clin Oncol. 2013;31:3026–3033.
- Frumovitz M, Querleu D, Gil-Moreno A, et al. Lymphadenectomy in locally advanced cervical cancer study (LiLACS): phase III clinical trial comparing surgical with radiologic staging in patients with stages IB2-IVA Cervical Cancer. J Minim Invasive Gynecol. 2014;21:3–8.
