Abstract
Background: Survival of patients with high-grade osteosarcoma (HOS), the most common primary bone cancer, has not improved significantly the last 30 years and the disease remains a major challenge. The purpose of this study is to evaluate survival in relation to prognostic factors at time of diagnosis among patients diagnosed with primary appendicular HOS in East Denmark between 1990 and 2010.
Material and methods: 101 patients (median age = 20 years, female/male ratio = 56/45) diagnosed with primary appendicular high-grade osteosarcoma between 1990 and 2010 were included in this study. Initially, 156 patients diagnosed with osteosarcoma between 1990 and 2010 were identified through the population based Regional Database of Pathology, which covers a population of approximately 2.7 million (east Denmark). 55 patients were excluded due to (A) tumor was low grade (n = 22), (B) located in axial skeleton (n = 18), (C) incorrect diagnosis (n = 11) or (D) biopsy represented a tumor relapse from a former primary osteosarcoma (n = 4). Overall survival was evaluated using the Kaplan–Meier survival analysis and log-rank test. Prognostic factors were analyzed using uni- and multivariate cox-regression method with variables scored equally in the model. p Values <.05 were considered statistically significant.
Results: The probability of 5- and 10-year survival was 51% (95% CI: 41–61) and 46% (95% CI: 36–56), respectively. Metastatic stage at diagnosis and tumor size ≥10 cm measured radiologically at the largest diameter were independent prognostic factors for decreased survival with significant increased hazard-risks of 3.5 (95% CI: 1.9–6.5) and 1.97 (95% CI: 1.1–3.6), respectively.
Discussion: In this single institution evaluation of primary appendicular HOS we found 5-and 10-year survival rates consistent with international standards for this patient group. Distant metastases and tumor size ≥10 cm at the time of diagnosis were independent prognostic factors for decreased survival in our cohort. These results underline the importance of awareness and early referral from the primary sector.
Introduction
Osteosarcoma is the most common primary malignant bone tumor with an overall age-standardized incidence rate of 2.9 per million in males and 2.2 per million in females [Citation1]. Approximately 90% of osteosarcomas are classified as high-grade and these are further classified by histology among which the classic primary appendicular high-grade osteosarcoma is the most frequent [Citation2].
High-grade osteosarcomas arise from mesenchymal cells most often in the long bones of the knee-region or proximal humerus. [Citation3]. Incidence of the disease peaks at adolescent age (10–14 years) and again around 70–80 years of age [Citation4] and recent studies present overall 5-year survival for high-grade osteosarcoma of approximately 45–75% depending on various prognostic factors [Citation2,Citation5–7].
Many different prognostic factors have been suggested to predict the survival outcome of high-grade osteosarcomas such as distant metastasis, primary tumor size, proximal tumor location, male gender, pathological fracture and age [Citation7,Citation8]. However, most recent studies agree on two significant independent high-risk variables at the time of diagnosis: (A) age ≥40 years [Citation7,Citation9] and (B) presence of metastases (observed in 20% of the patients, most often located to the lungs [Citation5]).
Following the implementation of chemotherapy and limb-salvage surgery in the 1970s and 1980s, the survival rate of high-grade osteosarcoma has increased significantly [Citation8]. However, no significant improvement in survival has been observed in the last three decades [Citation4,Citation10]. Accordingly, treating primary high-grade osteosarcoma remains a major multi-disciplinary challenge.
In this study, we present a single institution retrospective evaluation of 101 patients treated for primary appendicular high-grade osteosarcoma between 1990 and 2010 with a minimum of 5 years of follow-up since the time of diagnosis. We investigated patient demographics and tumor characteristics in order evaluate survival in relation to prognostic factors at time of diagnosis for this rare malignant disease.
Material and methods
Patient selection and treatment modalities
Between 1990 and 2010 all patients in east Denmark with suspicion of malignant bone disease were referred to the regional sarcoma center with primary referral to either the Department of Oncology, Department of Pediatric oncology or the Department of Orthopedic Tumor Surgery covering a background population of approximately 2.7 million. If suspicion was confirmed by a multidisciplinary team (radiologist, pathologist, oncologist, pediatrician and orthopedic surgeon), an open biopsy or an ultrasound guided core needle biopsy was performed. Subsequently, the sample was pathologically evaluated by a local team of specialized sarcoma pathologists and registered in a local database and in The Danish Database of Pathology [Citation11].
During the 21-year period of inclusion, the treatment of high-grade osteosarcoma evolved at our center in accordance with the changing treatment protocols. However, the overall treatment regimen was generalized throughout the study period. Overall, every single patient was treated by a multidisciplinary team in accordance with international protocols. In majority of cases, but depending on the individual patient and a multidisciplinary specialist’s assessment, this treatment regimen included: neoadjuvant- and/or adjuvant chemotherapy followed by surgery with the aim of obtaining a wide excision (either by limb-salvage or amputation surgery). The age group below 15 years of age received combination chemotherapy with cisplatin, doxorubicin and high-dose methotrexate (HD-MTX) during the entire study period, while patient’s older than 15 years received cisplatin and doxorubicin only until 2010 where HD-MTX was added to the treatment of this patient group [Citation12].
Data collection and creation of subgroups
A retrospective analysis of patient demographics, clinical information and tumor characteristics was performed. Data on gender, age at diagnosis, duration of symptoms, anatomic site, pathological fracture at the time of diagnosis, stage at diagnosis and size measured radiologically at the widest diameter of the primary tumor at the time of diagnosis were extracted from patients’ medical records and The Danish Database of Pathology. Data were collected during the spring of 2016 and vital status (obtained from The Danish Civil Registration system [Citation13]) was evaluated the 1 February 2016, resulting in a minimum follow-up period of 5 years for all patients included in the study.
Data were divided into subgroups in accordance with recent literature regarding prognostic factors for survival of high-grade appendicular osteosarcoma registered at time of diagnosis [Citation1,Citation2,Citation8]: Age was divided into three groups; <15 years, 15–40 years and ≥40 years, respectively. Tumor size was divided into two groups of <10 cm or ≥10 cm at the widest diameter (evaluated at the time of diagnosis by X-ray, magnetic resonance imaging or computer tomography). Finally, stage at diagnosis was investigated and registered as either local or disseminated.
Other plausible prognostic factors and subgroups investigated were: pathological fracture at time of diagnosis, duration of symptoms above four months and time of diagnosis divided into two periods (1990–1999 and 2000–2010) to investigate possible differences in survival between these two time periods. Finally, the cohort was divided according to gender and anatomic site of the tumor – upper/lower extremities ().
Table 1. Characteristics at time of diagnosis in 101 patients diagnosed with primary appendicular high-grade osteosarcoma in east Denmark between 1990 and 2010.
Statistics
Overall survival and prognostic factors for survival among variables known at time of diagnosis were investigated in this study. Overall survival was registered from time of diagnosis to time of death. If death did not occur in the examination period, the patient was censored (registered as alive) at the 1 February 2016. Survival was calculated using the Kaplan–Meier survival analysis with log-rank test, and prognostic factors were analyzed using uni- and multi-variate cox-regression method with variables scored equally in the model to present a hazard ratio for the given analysis. p Values <.05 were considered statistically significant. SPSS version 23 and SAS Enterprise 7.1 software were used for statistical assessments.
Ethical approval
This study was approved by the Danish Health Authority (3-3013-1533/1) and The Danish Data Protection Agency (2013-41-2225). All procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983.
Results
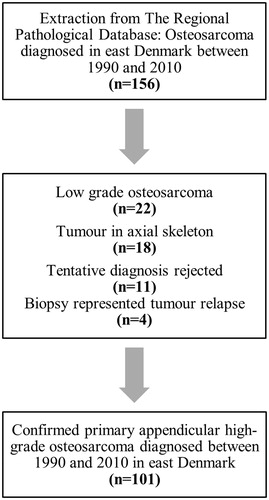
Between 1990 and 2010, 156 patients with a histological diagnosis of osteosarcoma were identified through the local pathology database. During this period, the population of the catchment area of our orthopedic oncology unit included a background population of approximately 2.7 million. Subsequently, all 156 patients’ medical records were studied to include only patients with primary appendicular high-grade osteosarcoma. In total, 55 patients were excluded due to the tumor being either low-grade (n = 22), located in the axial skeleton (n = 18), incorrect diagnosis (n = 11) or if the biopsy represented a tumor relapse from a previous primary osteosarcoma (n = 4). The remaining 101 patients with primary, appendicular, histologically confirmed high grade osteosarcoma were considered eligible for further analysis ().
Figure 1. Flowchart of patient selection: Primary appendicular high-grade osteosarcoma diagnosed in east Denmark between 1990 and 2010.
Patient characteristics
Primary appendicular high-grade osteosarcoma most frequently occurred in the lower extremities with a median size of 10 cm in diameter (range: 2.5–34.5 cm). Gender was almost evenly distributed in the population with a slight overweight of female patients (55.4%). Approximately one fifth of the patients presented with a pathological fracture and the median duration of symptoms before diagnosis was four months with a significant variation (0.5–40 months) ().
Sixteen patients (15.8%) had metastases at the time of diagnosis, five of whom had resistant disease. Among the remaining 85 patients (84.2%) who initially had localized tumors, 31 (36.5%) experienced metastatic relapse during follow-up. In total, 16 patients regardless of stage at diagnosis had local relapse during follow-up among which 10 patients had concomitant distant metastases.
Survival and prognostic factors
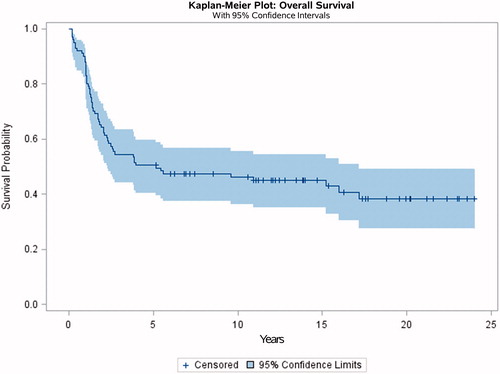
Mean follow-up after diagnosis was 11.45 years (range: 0.2–24 years). The cumulative 5- and 10-year survival probability for all 101 patients from the time of diagnosis was 51% (95% CI: 41–61) and 46% (95% CI: 36–56), respectively ().
Figure 2. Kaplan–Meier survival curve of all high-grade appendicular osteosarcoma diagnosed in east Denmark between 1990 and 2010.
In both the uni- and multi-variate analysis, metastatic disease at the time of diagnosis and tumor size ≥10 cm was associated with a statistically significant poorer survival and increased hazard for death in the first 5 years after diagnosis. Patients with metastatic disease at the time of diagnosis had a statistically significant lower 5-year cumulative survival probability and increased risk of dying within the first 5 years (hazard ratio = 3.5). The chance of surviving with localized disease at the time of diagnosis was 56% compared to 19% with metastatic disease ( and ).
Figure 3. Kaplan–Meier survival curves of prognostic factors (A: Stage at diagnosis and B: Tumor size at time of diagnosis) for survival and corresponding p value.
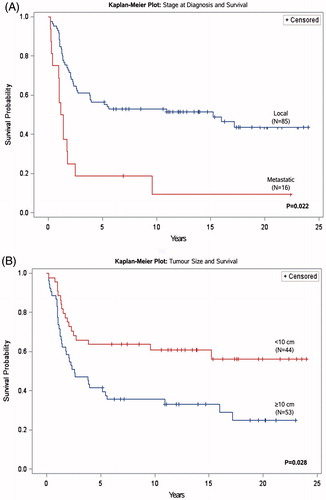
TABLE 2. Five-year survival and cox-regression analysis (univariate/multivariate) of prognostic factors at the time of diagnosis in all primary appendicular high-grade osteosarcoma diagnosed in east Denmark between 1990 and 2010.
Patients with a primary tumor ≥10 cm in diameter at the time of diagnosis had almost twice the risk of dying within 5 years as demonstrated by the statistically significant hazard ratio of 1.97 (). These patients also had a decreased 5-year survival probability of 42% compared to 64% for patients with smaller primary tumors ( and ).
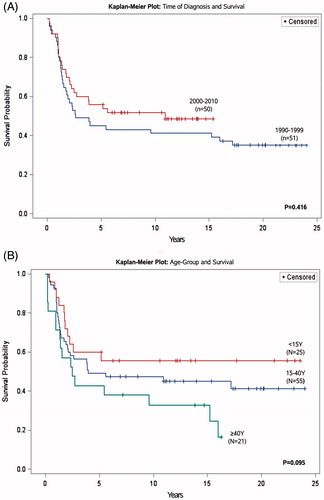
Among the other variables investigated in this study (), we found no statistically significant prognostic associations to survival. However, tendencies were observed in the cohort: patients diagnosed between 1990 and 1999 displayed a lower survival probability; p = .416, log-rank test, when compared to patients diagnosed between 2000 and 2010 ( and ) and among the three age-groups analyzed in this study, children of <15 years of age presented the highest 5- and 10-year survival probability; p = .095, log-rank test ( and ).
Discussion
In this study, we present a single center 21-year evaluation of survival in relation to prognostic factors at the time of diagnosis among patients with primary appendicular both localized and metastatic high-grade osteosarcoma. We found an overall cumulative 5- and 10-year survival rate of 51% (95% CI: 41–61) and 46% (95% CI: 36–56%), respectively, with a mean follow-up of 11.45 years (0.2–24). In current literature, there is an agreement of metastatic osteosarcoma being associated to decreased survival – investigated in both retrospective single-center observational studies [Citation2,Citation3,Citation5,Citation14] and larger reviews [Citation8,Citation15]. This is in accordance with the results of this study where we found that 5- and 10-year survival probabilities were 10–25% and the risk of death within 5 years was 2- to 8-fold higher among patients with disseminated disease at time of diagnosis compared to non-metastatic cases.
Other studies also investigating survival in cohorts of patients with both localized and metastatic disease, have reported 5-year survival rates of 42–55% which is comparable to our results [Citation1–3]. Among localized high-grade appendicular osteosarcomas we found in our study a 5-year survival-rate of 56% (95% CI: 46–76). This is in line with The European Intergroup Osteosarcoma study who found a similar 5-year survival-rate of 56% in their cohort of 1067 localized high-grade appendicular osteosarcomas in patient’s ≤40 years of age [Citation7]. However, 5-year survival-rates of localized high-grade appendicular osteosarcomas up to 80% have been reported in the first results of the Euramos-1 protocol as well as in other studies [Citation4,Citation12,Citation15].
In agreement with our findings, tumor size has previously been suggested as a prognostic factor by several authors, who report that size above 5–10 cm leads to poorer prognosis and decreased survival rate [Citation8,Citation9,Citation16]. Other studies have shown that primary tumor size can be closely related to the anatomic site of the tumor and that particularly axial tumors tend to be larger when diagnosed [Citation8] and thus related to decreased survival rate. Registering tumor size, however, has often been a difficult task due to both the surgical procedure, the performance of imaging devices and size heterogeneity of tumors in different bone types and between genders. Recent studies are currently investigating new methods to determine tumor size to predict prognosis in high-grade osteosarcoma [Citation17].
The need for further investigation in optimizing the diagnosis and treatment of high-grade osteosarcoma is warranted as no significant improvement in overall survival has been observed in the last two/three decades [Citation4,Citation10]. This agrees with the findings of our study. However, a tendency was observed in our study – as well as in other studies – that overall survival seems to be improving over time [Citation15].
Although tendencies were observed, no other statistically significant prognostic factors were detected when investigating age-groups, period of diagnosis, duration of symptoms, gender, pathological fracture or anatomic site of the primary tumor. This could be due to the relative small cohort of this study, making the separate subgroups too small to draw any statistically significant conclusions.
It is debated if age at the time of diagnosis, more specifically age ≥40 years is a prognostic factor for poorer survival and generally children and adolescents under the age of 15 at time of diagnosis have better survival-rate than adults and elderly [Citation2,Citation18]. Our results suggests a tendency toward survival being dependent on age but no statistically significant associations were detected when comparing overall survival in the age-groups listed in and illustrated in . However, this tendency may be related to the difference in the chemotherapy regimens applied in that chemotherapeutic protocols differ between children and adults as well as the lack of power in this study as discussed earlier.
Other studies present an age-related mortality; the younger the patient, the lower the 5-year mortality rate, due to the older patients’ inability to tolerate chemotherapy and/or a higher number of axial osteosarcomas, which might have a worse prognosis [Citation8].
We chose not to include axial high-grade osteosarcoma in this study due the fact that these patients were not systematically referred to our multidisciplinary center and therefore would propose a significant risk of bias if investigated and compared to the cohort of appendicular osteosarcoma. Whether axial and appendicular tumors represent significant differences in terms of survival potential remain unclear. Some studies suggest that there is a confounding potential in comparing these two types of high-grade osteosarcoma [Citation7,Citation15] because of the overweight of elderly patients with axial tumors [Citation8,Citation15] or that axial tumors might be more malignant [Citation16]. In contrast, other studies have found no statistically significant differences in survival when comparing axial and appendicular high-grade osteosarcomas [Citation2,Citation5].
In terms if anatomy, when investigating solely appendicular high-grade osteosarcomas, we were unable to find any statistically significant correlation between anatomic site and survival rate within our relatively small cohort of patients.
In a systematic review and meta-analysis from 2014, Sun et al. suggests that pathological fracture at the time of diagnosis predicts poorer survival compared to patients with no pathological fracture [Citation19]. A possible explanation for this could be that pathological fracture is associated with more advanced disease. In our study, we found no such association, which is in line with a another recent systematic review [Citation20].
Some studies found that female gender was associated with a statistically significant improved survival [Citation7,Citation21]. However, we found no associations between gender and survival and these findings agree with other studies as well [Citation2]. We and others [Citation5,Citation22] have been unable to show any relation between duration of symptoms and patient’s survival rate. However, this may be because symptoms duration is subjective, difficult to assess and could be influenced by factors such as recall bias, patient age and anatomical location.
The strengths of this study is that all patients were treated at the same multidisciplinary sarcoma center in Copenhagen, Denmark and all histopathology biopsy reports, regardless where the initial biopsy were taken, were reviewed and/or revised if necessary by a single team of sarcoma specialists at Rigshospitalet. Due to the Danish Civil Registration system [Citation13], we encountered no cases of loss to follow-up with regards to vital status.
A disadvantage of this study is the long inclusion period in which various treatment improvements occurred (i.e. better surgical technique and chemotherapeutic regime). This is due to advances in research and technology, especially with regards to surgical techniques and radiological equipment. HD-MTX was used as chemotherapeutic treatment in all patients of 15 years or younger in this study. In adults though, HD-MTX was not implemented before 2010. However, these changes did not significantly affect survival probability when comparing patients diagnosed between 1990–1999 and 2000–2010. This study is based on a relatively small cohort of patients diagnosed with both localized and metastatic high-grade appendicular osteosarcoma in east Denmark between 1990 and 2010 and we found 5- and 10-year survival rates of 51% (95% CI: 41–61) and 46% (95% CI: 36–56%), respectively. This finding is consistent with international survival outcomes for this disease however better results exists for subgroups of local stage at diagnoses and various age-groups.
Metastases at time of diagnosis and tumor-size ≥10 cm were independent statistically significant prognostic factors for decreased survival in our cohort, underlining the importance of awareness and early referral from the primary sector. No statistically significant improvement in survival was evident between times of diagnosis, emphasizing the need to continuously evaluate the treatment regimens and outcomes.
Current literature has yet to find common consensus regarding prognostic factors for survival in treating this difficult disease and further studies are warranted.
Acknowledgments
Authors thank Doctor Søren Daugaard, Chief Pathologist, Department of Pathology, Rigshospitalet, Copenhagen, Denmark.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Whelan J, McTiernan A, Cooper N, et al. Incidence and survival of malignant bone sarcomas in England 1979–2007. Int J Cancer. 2012;131:E508–E517.
- Aggerholm-Pedersen N, Maretty-Nielsen K, Keller J, et al. The importance of standardized treatment in high-grade osteosarcoma: 30 years of experience from a hospital-based database. Acta Oncol. 2015;54:17–24.
- Zaikova O, Sundby Hall K, Styring E, et al. Referral patterns, treatment and outcome of high-grade malignant bone sarcoma in Scandinavia–SSG Central Register 25 years' experience. J Surg Oncol. 2015;112:853–860.
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543.
- Berlanga P, Canete A, Diaz R, et al. Presentation and long-term outcome of high-grade osteosarcoma: a single-institution experience. J Pediatr Hematol Oncol. 2015;37:e272–e277.
- Durnali A, Alkis N, Cangur S, et al. Prognostic factors for teenage and adult patients with high-grade osteosarcoma: an analysis of 240 patients. Med Oncol. 2013;30:624.
- Whelan JS, Jinks RC, McTiernan A, et al. Survival from high-grade localised extremity osteosarcoma: combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol. 2012;23:1607–1616.
- Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47:283–292.
- Berner K, Hall KS, Monge OR, et al. Prognostic factors and treatment results of high-grade osteosarcoma in norway: a scope beyond the "classical" patient. Sarcoma. 2015;2015:516843.
- Venkatramani R, Murray J, Helman L, et al. Risk-based therapy for localized osteosarcoma. Pediatr Blood Cancer. 2016;63:412–417.
- Erichsen R, Lash TL, Hamilton-Dutoit SJ, et al. The Danish National Pathology Registry and Data Bank. Clin Epidemiol. 2010;2:51–56.
- Bielack SS, Smeland S, Whelan JS, et al. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance PEGylated interferon Alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol. 2015;33:2279–2287.
- Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549.
- Hung GY, Yen HJ, Yen CC, et al. Experience of pediatric osteosarcoma of the extremity at a single institution in Taiwan: prognostic factors and impact on survival. Ann Surg Oncol. 2015;22:1080–1087.
- Luetke A, Meyers PA, Lewis I, et al. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev. 2014;40:523–532.
- Meazza C, Luksch R, Daolio P, et al. Axial skeletal osteosarcoma: a 25-year monoinstitutional experience in children and adolescents. Med Oncol. 2014;31:875.
- Kim SH, Shin KH, Park EH, et al. A new relative tumor sizing method in epi-metaphyseal osteosarcoma. BMC Cancer. 2015;15:284.
- Iwata S, Ishii T, Kawai A, et al. Prognostic factors in elderly osteosarcoma patients: a multi-institutional retrospective study of 86 cases. Ann Surg Oncol. 2014;21:263–268.
- Sun L, Li Y, Zhang J, et al. Prognostic value of pathologic fracture in patients with high grade localized osteosarcoma: a systemic review and meta-analysis of cohort studies. J Orthop Res. 2015;33:131–139.
- Cates JM. Pathologic fracture a poor prognostic factor in osteosarcoma: Misleading conclusions from meta-analyses? Eur J Surg Oncol. 2016;42:883–888.
- Min D, Lin F, Shen Z, et al. Analysis of prognostic factors in 333 Chinese patients with high-grade osteosarcoma treated by multidisciplinary combined therapy. Asia-Pac J Clin Oncol. 2013;9:71–79.
- Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann Oncol. 2015;26:407–414.