Introduction
Lung cancer remains the most common and deadly cancer worldwide [Citation1]. Non-small cell lung cancer (NSCLC) accounts for about 85% of lung cancers, where locally advanced (LA) disease accounts for approximately 35% of these patients [Citation2]. Although concurrent chemoradiotherapy (cCRT) improves locoregional control and survival compared to the sequential approach (sCRT), the clinical outcome of CRT for LA-NSCLC could be refined [Citation2,Citation3]. In recent years, several strategies have been studied to improve the therapeutic ratio, including altered fractionation [Citation4] and dose escalation [Citation5] with disappointing results. Even though the optimal radiation dose during CRT is still unknown [Citation6], recent technological improvements (image guidance, advanced delivery techniques) could still be further explored towards individualized treatment approaches such as personalized isotoxic dose escalation to the residual [Citation7] or 18F-fludeoxyglucose–positron emission tomography/computed tomography (FDG-PET/CT) avid [Citation8] tumor, adapting the treatment fields to the shifting [Citation9] or shrinking tumor [Citation10].
To reach full potential the use of image-guided radiotherapy (IGRT), e.g., by cone-beam computed tomography (CBCT) [Citation11] could be used to monitor tumor and organs at risk (OAR) volume and/or position changes over the treatment course. This could ensure adequate target coverage while improving treatment outcome [Citation12–15]
Several studies report on imaging during radiotherapy (RT) to evaluate tumor volume shrinkage and to predict the outcome, in order to identify patients benefiting from treatment intensification. As the primary tumor volume shrinks quasi-linearly with additional fractions, predicting final volume decrease at early stage is feasible and adaptation of the treatment plan (adaptive RT, ‘ART’) at the optimal timepoint at fraction 15 and 20 for cCRT and sCRT, respectively [Citation16] could lead to lower doses to the OAR. However, the potential underdosage of microscopic disease after ART remains a genuine concern, particularly in case of intensity modulated radiotherapy (IMRT).
In this retrospective follow-up study, we aimed to quantify tumor and involved lymph node volume changes during cCRT and sCRT after 15 and 20 fractions respectively and determine the gain on OAR, while evaluating the risk of underdosage within the microscopic disease of ART. Furthermore, we aimed to investigate effect of the tumor shrinkage at the time of the optimal adaptation on outcome.
Material and methods
Forty-one consecutive patients treated with (three cycles of platinum-based) cCRT or sCRT were included in the study. All patients were treated with IMRT [Citation17] up to 70 Gy (range: 62–70 Gy) in 2 Gy per fraction. Population, baseline patient and tumor characteristics are presented in Supplementary Table 1.
Target delineation and imaging protocol have been previously described [Citation16]. In brief, the initial gross tumor volume (GTV-T_init) and the involved lymph nodes (GTV-Lnn_init) were delineated on the planning CT and FDG-PET/CT [Citation18]. Initial GTV’s were summed to create a GTV_tot_init and expanded by 5 mm to create a clinical target volume (CTV_tot_init), which was adapted to exclude normal structures. A 5 mm margin was added towards the planning target volume (PTV_tot_init). At every treatment fraction, a CBCT scan was performed (OBI, 110 kVp, 1mA/s, Varian Medical Systems, Palo Alto, CA, USA). CT-CBCT was co-registrated by trained radiation therapists, supervised by the radiation oncologist (RO). Six-degree co-registration on bony anatomy match was performed and used to translate to optimal three-degree translational daily table correction. Emphasis of the registration was towards accurate match on the tumor and surrounding lung. The used registrations with the CBCTs were automatically imported to the treatment planning system (Eclipse version 8.9, Varian Medical Systems, Palo Alto, CA). At fraction 15/20 for c-/s-CRT respectively, a GTV_tot_adap was created on the corresponding CBCT: Adaptation of GTV_tot_init was performed only in regions where previously present macroscopic tumor tissue visually disappeared and lymph nodes retracted towards the mediastinum. All delineations were performed by one RO (P.B.) to limit inter-observer variability. OAR volumes were not adapted. We addressed the density change systematically: the part of the GTV_tot_init outside the GTV_tot_adap (i.e., the disappearing tumor volume after shrinkage) was identified and assigned with a 700 Hounsfield Unit density for the re-optimization [Citation19].
For 35 out of 41 patients, three dose matrices were generated: the initial, the full (planned for the entire course of RT) and the actual adaptive plan using fraction-based summation of initial and adapted plans after adaptation. During the re-optimization for the full adaptive plan, beam arrangements, objectives for the corresponding target volumes and OARs remained the same. The relevant dose volume parameters were analyzed using two-sided t-test.
The potential underdosage of microscopic disease (MD) in a worst case scenario – whereby the potential MD area (i.e., CTV_tot_init minus GTV_tot_init) remains stationary – was simulated [Citation20]. Accumulated doses to this area were calculated taking into account the initial and adaptive plan, enabling us to evaluate dose coverage of the MD area via the CTV_tot_init (using the D95% parameter).
As the tumor shrinkage is quasi-linear during RT [Citation16], the population was split according to the primary GTV (GTV-T) above and below median volume reduction (higher and lower gradient changes). Impact of the tumor regression gradient on local control (LC), lung and distant progression free survival (lung and distant PFS), overall and disease specific survivals (OS and DSS) were also assessed based on the original treatment using the Kaplan–Meier survival comparison (log-rank test, p < .05 were considered significant). Further survival analyses were performed using chemotherapy type, age, histology and gender as input parameters.
Results
Median shrinkage from init to adap was 26% (Range [−25%, 75%]) for GTV-T, 19% (Range [0%, 70%]) for GTV_tot, while for CTV_tot and PTV_tot 12% (Range [−9%, 56%]) and 11% (Range [−7%, 50%]) respectively at the optimal time of adaptation (Supplementary Figure S1) After re-optimization, the differences between the corresponding PTV coverage parameters (D98% and V95) showed no significant difference (, p = .12 and .42), thus the observed gains on OAR doses originate from the tumor shrinkage. Representative DVH parameters of the OARs were statistically significantly (p < .05) lowered by adaptation. The consequent dose to the MD (using the D95 of CTV_tot_init) was originally 66.1 (SD:2.1) and after adaptation 65.1 Gy (SD:3.1) (). The median follow-up was 9.8 months (0.2–64.7). Cutoff of shrinkage gradient for GTV-T was 23%, which showed statistically significant impact on lung PFS and DSS (p = .04 and .02, respectively, ). Furthermore, age affected the distant PFS (p = .02, Supplementary Figure S2).
Figure 1. Kaplan-Meier survival curves (with 95% CI and p-values from the log-rank test) on outcome based on the GTV-T shrinkage gradient.
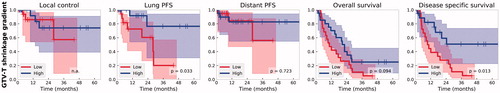
Table 1. Dose volume histogram parameters from the initial and adaptive, and the fraction-based combination (sum adaptive).
Discussion
In this retrospective study, we implemented the previously proposed ART strategy [Citation16]. Using CBCT to re-shape the GTV-T shows excellent agreement with CT [Citation21], however for GTV-Lnn, the CBCT image lacks contrast to distinguish between lymph node and other surrounding tissue, thus adaptation is only feasible on the lung-lymph node boundary. Lymph node shrinkage on CT during CRT has been reported ranging from 0% to 37% for the complete treatment [Citation22–25] compared to our results of 17% until the optimal time of adaptation using CBCT only, contributing to 8% of the total shrinkage. In summary, early treatment response evaluation of the involved lymph nodes (on CBCT as well) is not validated in contrast to the GTV-T.
The mean tumor volume shrinkage of 1.6%/day is in good agreement with previously published results of 0.6–2.4%/day [Citation26]. This decrease translated into a statistically significant decrease in doses to OARs using ART.
In fact, the heart V5 and V30 decreased, providing a potential towards overall survival benefit, based on the RTOG 0617 [Citation5,Citation27]. Even though in our cohort lower heart dose did not show a survival benefit (Supplementary Figure S3), further large cohort pooled analyses including ART may provide evidence towards dose escalation and/or lowering the dose to the heart [Citation28].
Our data confirm significant OAR dose reduction without compromising initial CTV coverage, as the MD area remained adequately covered, well above the required 50 Gy [Citation20].
We observed that higher gradient shrinkage leads to statistically significantly improved lung PFS and DSS, while others reported overall survival benefit [Citation29]. This might be explained by the difference in cohorts (only cCRT versus mixed c-/s-CRT)
Taking all aspects into consideration, a safe ART using tumor shrinkage gradient for NSCLC patients based on CBCT evaluation could be proposed, as it is a viable option. This could be used to predict final tumor shrinkage and dosimetric gains at 15/20 fractions for c-/s-CRT. Furthermore, this decline also affects patient outcome, thus it could be exploited towards isotoxic dose escalation on the shrinking tumor and/or further OAR dose reduction.
Limitations of the current study are the small cohort and the omission of breathing motion. Another potential shortcoming is the delineation of involved lymph nodes on CBCT, in comparison to ‘gold standard’ anatomical planning CT.
Based on our tumor shrinkage adaptive radiotherapy, significant OAR dose reduction is feasible without compromising initial CTV coverage. Improved outcome is observed for patients with higher tumor shrinkage gradient.
IONC_A_1352103_Supplementary_Information.zip
Download Zip (1.8 MB)Acknowledgments
Dr Katrien Vandecasteele holds a mandate for fundamental and clinical research of the Foundation against Cancer – Belgium.
Disclosure statement
The authors report no conflicts of interest.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190.
- Eberhardt WEE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol. 2015;26:1573–1588.
- Haslett K, Pöttgen C, Stuschke M, et al. Hyperfractionated and accelerated radiotherapy in non-small cell lung cancer. J Thorac Dis. 2014;6:328–335.
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial. Lancet Oncol. 2015;16:187–199.
- Ramroth J, Cutter DJ, Darby SC, et al. Dose and fractionation in radiation therapy of curative intent for non-small cell lung cancer: meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2016;96:736–747.
- Zhang P, Rimner A, Yorke E, et al. A geometric atlas to predict lung tumor shrinkage for radiotherapy treatment planning. Phys Med Biol. 2017;62:702–714.
- Kong F-M, Ten Haken RK, Schipper M, et al. Effect of midtreatment PET/CT-adapted radiation therapy with concurrent chemotherapy in patients with locally advanced non-small-cell lung cancer. JAMA Oncol. 2017;61:318–328.
- Tvilum M, Khalil AA, Møller DS, et al. Clinical outcome of image-guided adaptive radiotherapy in the treatment of lung cancer patients. Acta Oncol. 2015;54:1430–1437.
- Guckenberger M, Wilbert J, Richter A, et al. Potential of adaptive radiotherapy to escalate the radiation dose in combined radiochemotherapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:901–908.
- Korreman S, Rasch C, McNair H, et al. The European Society of Therapeutic Radiology and Oncology-European Institute of Radiotherapy (ESTRO-EIR) report on 3D CT-based in-room image guidance systems: a practical and technical review and guide. Radiother Oncol. 2010;94:129–144.
- Kwint M, Conijn S, Schaake E, et al. Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy. Radiother Oncol. 2014;113:392–397.
- Knap MM, Hoffmann L, Nordsmark M, et al. Daily cone-beam computed tomography used to determine tumour shrinkage and localisation in lung cancer patients. Acta Oncol. 2010;49:1077–1084.
- Persoon LC, Egelmeer AG, Ollers MC, et al. First clinical results of adaptive radiotherapy based on 3D portal dosimetry for lung cancer patients with atelectasis treated with volumetric-modulated arc therapy (VMAT). Acta Oncol. 2013;52:1484–1489.
- Kilburn JM, Soike MH, Lucas JT, et al. Image guided radiation therapy may result in improved local control in locally advanced lung cancer patients. Pract Radiat Oncol. 2016;6:e73–e80.
- Berkovic P, Paelinck L, Lievens Y, et al. Adaptive radiotherapy for locally advanced non-small cell lung cancer, can we predict when and for whom? Acta Oncol. 2015;54:1438–1444.
- Derycke S, De Gersem WR, Van Duyse BB, et al. Conformal radiotherapy of Stage III non-small cell lung cancer: a class solution involving non-coplanar intensity-modulated beams. Int J Radiat Oncol Biol Phys. 1998;41:771–777.
- De Ruysscher D, Faivre-Finn C, Nestle U, et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. JCO. 2010;28:5301–5310.
- Shin KE, Chung MJ, Jung MP, et al. Quantitative computed tomographic indexes in diffuse interstitial lung disease: correlation with physiologic tests and computed tomography visual scores. J Comput Assist Tomogr. 2011;35:266–271.
- Guckenberger M, Richter A, Wilbert J, et al. Adaptive radiotherapy for locally advanced non-small-cell lung cancer does not underdose the microscopic disease and has the potential to increase tumor control. Int J Radiat Oncol Biol Phys. 2011;81:e275–e282.
- Michienzi A, Kron T, Callahan J, et al. Cone-beam computed tomography for lung cancer – validation with CT and monitoring tumour response during chemo-radiation therapy. J Med Imaging Radiat Oncol. 2017;61:263–270.
- Juhler-Nøttrup T, Korreman SS, Pedersen AN, et al. Interfractional changes in tumour volume and position during entire radiotherapy courses for lung cancer with respiratory gating and image guidance. Acta Oncol. 2008;47:1406–1413.
- Pantarotto JR, Piet AHM, Vincent A, et al. Motion analysis of 100 mediastinal lymph nodes: potential pitfalls in treatment planning and adaptive strategies. Int J Radiat Oncol. 2009;74:1092–1099.
- Weiss E, Robertson SP, Mukhopadhyay N, et al. Tumor, lymph node, and lymph node-to-tumor displacements over a radiotherapy series: analysis of interfraction and intrafraction variations using active breathing control (ABC) in lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:e639–e645.
- Bosmans G, van Baardwijk A, Dekker A, et al. Time trends in nodal volumes and motion during radiotherapy for patients with stage III non-small-cell lung cancer. Int J Radiat Oncol. 2008;71:139–144.
- Sonke J-J, Belderbos J. Adaptive radiotherapy for lung cancer. Semin Radiat Oncol. 2010;20:94–106.
- Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62.
- Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non–small cell lung cancer. J Thorac Oncol. 2017;12:293–301.
- Jabbour SK, Kim S, Haider SA, et al. Reduction in tumor volume by cone beam computed tomography predicts overall survival in non-small cell lung cancer treated with chemoradiation therapy. Int J Radiat Oncol. 2015;92:627–633.
