Abstract
Background: Calcium electroporation is a novel anticancer treatment, which utilizes high voltage pulses to permeabilize cell membranes and expose the cell to supraphysiological doses of calcium. Preclinical studies on calcium electroporation have shown strikingly high tumor response with cell necrosis. Calcium electroporation builds on the treatment electrochemotherapy, where chemotherapeutic drugs, mostly bleomycin, are internalized by electroporation. This double-blinded randomized study compared calcium electroporation to electrochemotherapy in terms of objective response measured 6 months after treatment.
Methods: Seven patients with a total of 47 cutaneous metastases from breast cancer and malignant melanoma were included in the protocol. A total of 37 metastases were randomized and evaluated for response, another 10 metastases were used for biopsy. This was a non-inferiority trial and metastases were randomized individually in each patient to either intratumoral calcium or bleomycin followed by application of electric pulses to tumor site. All metastases were treated once, and after 6-months of follow-up, the randomization code was revealed.
Results: Objective response of calcium electroporation was 72% (13/18) with complete response in 66% (12/18). For electrochemotherapy, objective response was 84% (16/19) with complete response in 68% (13/19). There was no statistically significant difference between the two treatments (p = 0.5). After 1 year, only three out of 25 metastases had relapsed. Ulceration, itching and exudation were reported slightly more frequently in metastases treated with bleomycin, and hyperpigmentation was only seen in metastases treated with bleomycin.
Conclusion: This study shows that calcium electroporation is feasible and effective in patients with cutaneous metastases.
Introduction
Up to 9% of all cancer patients develop cutaneous metastases [Citation1] which can become exuding, bleeding and odorous, why there is a need for good palliative treatment. Electrochemotherapy describes the augmentation of cytotoxicity of chemotherapeutic agents such as bleomycin by permeabilization of tumor cells using brief, high voltage electric pulses (electroporation). Electrochemotherapy may be used for cutaneous metastases from all tumor histologies [Citation2–14]. It has consistently shown very high response rates after local tumor treatment, and has quickly gained acceptance in many European cancer centers.
Recently, introduction of supraphysiological doses of calcium by electroporation was described, and it was shown that this treatment modality led to a strikingly high tumor response with cell necrosis associated with acute and severe ATP depletion [Citation15,Citation16]. As calcium electroporation would have potential as a local treatment of cutaneous metastases, we decided to launch a double-blinded randomized controlled trial, to investigate if calcium electroporation would be able to produce responses similar to those observed for electrochemotherapy.
Patients and methods
Study design
This was a randomized double-blinded phase II study with a primary endpoint to compare tumor response of calcium electroporation with tumor response of electrochemotherapy with intratumoral bleomycin in patients with cutaneous metastases. The secondary endpoint was to evaluate toxicity of calcium electroporation. A tertiary endpoint was set to calculate whether the delivered current was affected in metastases treated with calcium chloride compared to metastases treated with bleomycin.
The protocol was approved by the Danish Medicine Agency, The Regional Ethics Committee and the Danish Data Protection Agency. The study was performed according to Good Clinical Practice (GCP). Clinicaltrials.gov identifier: NCT01941901.
Patients
Inclusion criteria were minimum of one histologically confirmed cutaneous metastasis of any histology and size from 0.5 to 3.0 cm. The patient should have been offered current standard treatment. Patients who received endocrine or targeted therapy, vinorelbine, capecitabine or weekly paclitaxel could continue these treatments if there was no regression of cutaneous metastases, otherwise at least 2 weeks since previous treatment was required. Patients had to be at least 18 years old, ECOG performance status ≤2 [Citation17] and have a life expectancy of at least 3 months. Platelet count ≥50E9/l and prothrombin time ≥40 s was required. Sexually active men and women with childbearing potential had to use adequate contraception during this trial. The patient had to be able to understand the information and to sign informed consent.
Exclusion criteria were previous bleomycin treatment with more than 200,000 IU/m2, history of severe allergic reactions associated with bleomycin, uncorrectable coagulation disorder, as well as pregnancy or lactation. Patients could not be included if they participated in other clinical trials involving experimental drugs or if they had been involved in a trial within 4 weeks prior to study drug administration.
Randomization
Randomization and evaluation was performed separately on each metastasis, so that each patient would potentially have both calcium electroporation and electrochemotherapy in different metastases. Block randomization was performed 1:1 separately into the two treatment arms.
A maximum of 10 metastases per patient were included. If the patient had 1–6 metastases, all metastases were randomized and evaluated for response. Biopsies (before and/or after treatment) were performed in patients with more than 6 metastases. If patients had more than 10 metastases, the remaining were treated with standard intratumoral bleomycin, but not included in the protocol.
The randomization lists were made by the Clinical Research Unit and the pharmacy mixed calcium chloride and bleomycin into syringes with appropriate dose for the calculated tumor volumes (). The treating doctors had no contact with the randomization or mixing of the drugs and as calcium chloride and bleomycin are colorless and volumes were identical, both doctors and patients were fully blinded to content of the syringes. It was a once-only treatment and after 6 months of follow-up, or when the patient was withdrawn from the trial, the randomization code was revealed.
Figure 1. Illustration of study design At first visit, metastases were numbered and measured. Data were then brought to an external unit (the pharmacy) who randomized and mixed calcium chloride and bleomycin into syringes labeled with numbers according to the metastases. At treatment, the blinded contents of the syringes were injected intratumorally and followed by electroporation. It was a once-only treatment and the patients were followed for 6 months with regular evaluation of tumor response and safety.
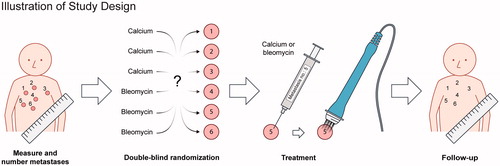
Previous treatments
For each metastasis, it was registered whether it was in a previously irradiated area or in an area with lymphedema. Concomitant treatments were recorded ().
Table 1. Patients demographics.
Calcium and bleomycin doses
The dose of calcium chloride was estimated from preclinical studies [Citation15–18] and set to 9 mg/ml (220 mmol/l). The bleomycin dose was set to 1000 IU/ml in accordance with European Standard Operating Procedures for Electrochemotherapy (ESOPE) [Citation19]. Initially, the injected volume for both bleomycin and calcium chloride were 0.5 ml/cm3 tumor volume. After treatment of five patients, the volume for the smaller tumors (≤0.5 cm3) was amended to 1 ml/cm3. Tumor volume was calculated as ab2π/6, a = largest diameter, b = largest diameter perpendicular to a.
A list of metastases with volumes was handed to the pharmaceutical unit and syringes labeled with metastasis number were returned, containing either calcium or bleomycin.
Procedure
The treatment was performed in local anesthesia (lidocaine with epinephrine) based on ESOPE [Citation19]. The content of the numbered syringes was injected intratumorally and pulses were delivered immediately after drug administration. Electrodes with two parallel rows of needles (linear array) were used, eight pulses of 0.1 ms duration, and 400 V at a frequency of 5 kHz were delivered using square wave pulse generator (Cliniporator, IGEA, Italy for the first five patients and Cliniporator Vitae IGEA, Italy for the last two patients).
Tumor response
Assessment of response was performed by clinical examination at day 15, 30, 60, 90, 180. Response was registered for each target lesion and documented by digital photography including a ruler. Response was evaluated similar to RECIST v.1.1 [Citation20] and defined as Complete Response (CR) – disappearance of the lesion, Partial Response (PR) – at least 30% decrease in the largest diameter of the lesion, Progressive Disease (PD) – at least 20% increase in the largest diameter of the lesion and Stable Disease (SD) – neither 30% decrease nor 20% increase of the largest diameter of the lesion. To evaluate long lasting response, the patients were examined again, if possible, 1 year after treatment.
Pathology
If the patients had more than 6 metastases, punch biopsies were collected from additional metastases before and/or after treatment (day 7). The biopsies were cut frozen and H&E stained to determine the level of tumor cells, necrosis, fibrosis and inflammation.
Safety assessment
Adverse events were recorded at the days of clinical examination using Common Toxicity Criteria for Adverse Events version 4.0 [Citation21], and pain was recorded using Numeric Rating Scale for pain (NRS, 0 is no pain, 1–3 is mild pain, 4–6 is moderate pain and 7–10 is severe pain) [Citation22] before and after treatment. As a measure of how patients felt about the procedure they were asked if they potentially would agree for another session.
Statistical analysis
This study was set up as a non-inferiority study between calcium electroporation and electrochemotherapy. Primary endpoint was to compare objective response (CR and PR) of the two treatments. After reviewing existing data from electrochemotherapy with intratumoral bleomycin on small cutaneous metastases (≤3cm), we estimated the expected response rate for electrochemotherapy to be 85% [Citation2–12]. There were no preexisting clinical data on calcium electroporation, but we decided to accept a difference in response of 15%, thus we estimated the expected objective response rate for calcium electroporation to be 70%. To determine the required number of metastases, we used a non-inferiority analysis and with a significance level of 0.05 and a power 80% the study required 34 evaluable metastases.
All statistical analyses were done using SPSS v22. Tumor response was analyzed using χ2-test on objective responses 6 months after treatment. Difference in highest measured current was analyzed using two tailed t-test with Bonferroni correction and for adverse events only descriptive statistics, as percentages, were used.
Results
Patients and metastases
Between October 2013 and February 2016, seven patients with a total of 47 metastases were included in the protocol, six patients had breast cancer and one patient had malignant melanoma. presents patient characteristics and previous and concomitant treatments at baseline.
Of the 47 included metastases, 37 metastases were randomized and evaluated for response and 10 metastases were used for biopsies. The median diameter of the 37 response evaluated metastases was 10 mm (range: 5–25 mm), and the characteristics of the metastases were equally distributed between the two treatment arms ().
Table 2. Metastases used for response evaluation.
Tumor response
Response rate at 6 months of follow-up showed for calcium electroporation an objective response of 72% (CR = 66%; PR = 5%) and for electrochemotherapy an objective response of 84% (CR = 68%; PR = 15%). There was no significant difference in objective response between calcium electroporation and electrochemotherapy after 6 months (p = 0.5) (B). The 2-sided 95% CI for the outcome difference between the 2 groups was 14.5%–38. 5% and did not cross the 15% preselected non-inferiority margin why the study proved non-inferiority for calcium electroporation.
Figure 2. Tumor response Metastases were treated at day = 0 with either i.t. calcium or i.t. bleomycin in a randomized double-blinded study design. The metastases were treated once only and after 6 months of follow-up, the randomization code was revealed. A. Change in diameter over time: The graph illustrates change in largest diameter over time for metastases treated with calcium chloride (red) and bleomycin (blue) (mean and standard deviation). The three non-measurable metastases treated with bleomycin were not included. B. Percent change in tumor size 6 months after treatment: The graph illustrates a clear difference in tumor response when subdivided into irradiated (CR = 46%) and non-irradiated (CR = 81%) metastases. The three non-measurable metastases treated with bleomycin were non-irradiated, and are not illustrated in the graph, but included in the calculation. C. Percent metastases without progression: The graph illustrates progression in treated metastases after treatment. After 6 months, 11% (2 out of 18) metastases treated with calcium had progressed, and 15% (3 out of 19) metastases treated with bleomycin had progressed. D. Highest measured current: The graph illustrates that there is no significant difference in measured current between bleomycin and calcium (p = 0.5), but a significantly higher delivered current in non-irradiated metastases (light grey) compared to irradiated metastases (dark grey) (p < 0.0001).
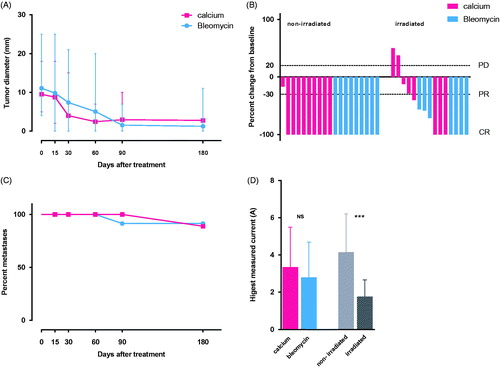
A significantly different response was observed between previous irradiated metastases and non-irradiated metastases as a larger proportion of metastases in non-irradiated skin showed complete response after 6 months (81% vs. 46%; p = 0,036).
In one patient, three of the metastases progressed into one coalescent, and verified malignant, tumor. The three metastases, all in the bleomycin group, were included in the response analysis as PD; however, measurement of the individual metastases could not be performed.
Five patients had follow-up for 1 year after treatment, and out of 25 metastases, three metastases had relapsed within a year after treatment. All three metastases were treated with calcium chloride.
Pathology
Punch biopsies from metastases were collected before treatment and 7 days post-treatment from different metastases. Pathology from both metastases treated with calcium electroporation and metastases treated with electrochemotherapy showed less tumor infiltration and more necrosis compared to untreated metastases ().
Figure 3. Punch biopsies before and one week after treatment. A–C: Patient no. 3, with nine metastases from breast cancer on the right nates. A: Pre-treatment biopsy: Skin biopsy with a moderate amount of tumor infiltration (black arrow) and fibrosis within the dermis. There is mild inflammation and no necrosis. B: Day 7. Post-treatment with calcium electroporation: Ulcerated skin biopsy with the same amount of tumor infiltration as in the pretreatment biopsy and moderate fibrosis. There is no inflammation or necrosis. C: Day 7. Post-treatment with electrochemotherapy: Ulcerated skin biopsy with no evidence of tumor but a large amount of necrosis (grey arrow) and fibrosis and with mild inflammation. D–F: Patient no. 5 with ten metastases from breast cancer on her left breast and abdomen. D: Pre-treatment biopsy: Skin biopsy with extensive tumor infiltration, moderate fibrosis and minimal inflammation. No evidence of necrosis. E: Day 7. Post-treatment with calcium electroporation: Skin biopsy with less tumor infiltration than in the pretreatment biopsy and a moderate amount of necrosis and fibrosis. There is minimal inflammation. F: Day 7. Post-treatment with electrochemotherapy: Skin biopsy with moderate tumor infiltration, necrosis and fibrosis. There is minimal inflammation.
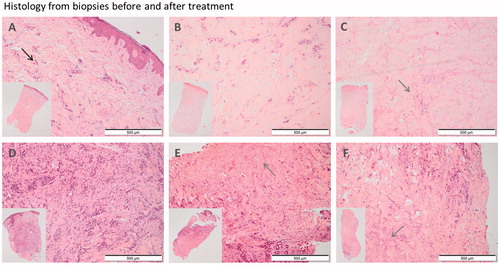
Safety
No serious adverse events were observed. Brief contractions of the underlying musculature were triggered by the electric pulses, similar for both treatments, causing brief discomfort in some patients. Otherwise, only CTC grade 1 was reported after treatment (). Ulceration, itching and exudation were reported slightly more frequently in metastases treated with bleomycin compared to metastases treated with calcium. A notable difference was seen in pigmentation, as only metastases treated with bleomycin appeared hyperpigmented after treatment (26%) none of the metastases treated with calcium had altered pigmentation ().
Figure 4. The top row show pictures from patient no. 2 with cutaneous metastases from breast cancer. Metastases 2 and 3 were treated with bleomycin and electroporation, metastasis 4 were treated with calcium and electroporation. The two metastases labeled B were treated with bleomycin outside of protocol. A. before treatment. B. Two weeks after treatment, shows crust on metastases no. 2, disappearing of metastases no. 3, and no visible chance in metastasis no. 4. C. Two months after treatment, show clear hyperpigmentation equivalent to the area treated with bleomycin (metastases no. 2 and 3) and no change in pigmentation in the area treated with calcium (metastases no. 4). Bottom row show pictures from patient no. 7 with cutaneous metastases from breast cancer. Metastases 1–3 were treated with bleomycin and electroporation and metastases 4–6 were treated with calcium and electroporation. D. before treatment. E. Two weeks after treatment, illustrates typical crust appearance, note the ulcer is not extending tumor margin. F. Six months after treatment, shows complete disappearance of metastases 1–5 and no change in metastasis no. 6.
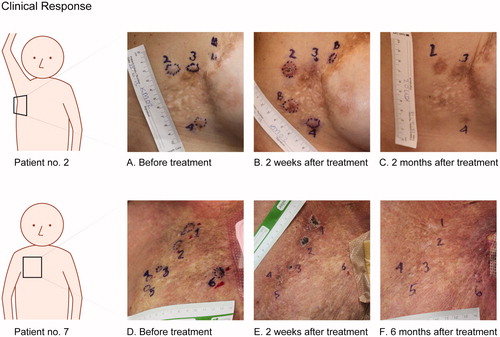
Pain was not assessed in individual lesions, but as an overall experience. In the period after treatment, four of the patients had no pain (NRS = 0), two patients reported mild pain (NRS = 1–3) and one patient reported moderate pain (NRS = 4–6). No patient wanted increase of pain medication.
Six out of seven patients expressed that they would agree to undergo the treatment again if necessary.
Treatment and delivered current
A total of 18 metastases were treated with calcium chloride and 19 metastases were treated with bleomycin. The median injected volume and the number of applied electric pulses were similar in the two treatment groups (). A tendency of a higher delivered current was measured in metastases treated with calcium chloride compared to metastases treated with bleomycin, but the difference was not significant. However, the measured delivered current was significantly higher in metastases located in non-irradiated skin compared to metastases located in previous irradiated skin (p < 0.0001) ().
Follow-up
Follow-up for 6 months regarding response evaluation was performed. One patient had progression of her cutaneous metastases after 5 months and was withdrawn from the protocol to start systemic chemotherapy (patient no. 5), her metastases were measured the day of her exclusion and the measurements were included in the final analysis. During the 6 months of response evaluation, the patients had either no concurrent treatment or persistent treatment with endocrine treatment or trastuzumab.
After the 6 months of response evaluation, additionally five patients had progression with new cutaneous metastases outside the treatment area. Two patients had a second session of electrochemotherapy (patient no. 1 and 2), three patients started systemic chemotherapy (patient no. 4, 5 and 7) and one patient had both a second session of electrochemotherapy and started chemotherapy (patient no. 3). One patient had complete regression and no further treatment, until progression 18 months after treatment (patient no. 6) [Citation23]. Five out of the seven patients had a long-term follow-up for 12 months after treatment, to monitor if there was recurrence in the treated area.
Discussion
In this first clinical study with calcium electroporation, we showed that calcium electroporation is safe and effective in treatment of small cutaneous metastases equally with electrochemotherapy in six patients with breast cancer and one patient with malignant melanoma, a group of patients with years of cancer history, and multiple treatments including surgery, radiotherapy and several regimes of systemic treatment.
Electrochemotherapy is an established treatment [Citation19] and has been used in treatment for cutaneous metastases for years and been encompassed in guidance by the National Institute for Health and Care Excellence (NICE) [Citation24]. Electrochemotherapy can be performed locally with intratumoral injection of bleomycin, and with intravenous injection of bleomycin. The latter is often preferred in larger treatment areas.
In contrast, calcium electroporation is a novel treatment where only a little experience is generated. However, preclinical studies with calcium electroporation have shown impressive results in cancer cell death and induction of tumor regression [Citation15]. Calcium is a vital intracellular signal molecule, dependent on a low, tightly regulated intracellular calcium concentration [Citation25], which is overrun by calcium electroporation. The more exact mechanism in how it leads to necrosis in cancer cells is still not completely clear, but may be associated to their lack of tolerance towards the sudden and severe ATP depletion [Citation15,Citation26,Citation27].
As calcium chloride is an approved drug accessible in the clinic, and the electroporation procedure well-integrated, it was possible to integrate the treatment relatively fast in a clinical protocol. Calcium electroporation may, in our opinion, only be injected locally in order to avoid systemic effects of the drug.
Response
In this study, we followed the guidelines from ESOPE [Citation19] according to applied voltage, pulses and drug injecting volumes for treatment using intratumoral injection, but used calcium chloride instead of bleomycin in half the number of metastases. Here, we confirm an objective response of 84%, similar to what previous clinical studies for electrochemotherapy for small cutaneous metastases have shown (84%–100%) [Citation2–14] and we saw no significant difference in objective response of calcium electroporation 6 months after treatment (p = 0.5). Biopsies collected 7 days after treatment also showed a markedly smaller number of cancer cells and higher level of cell death in both metastases treated with calcium electroporation and electrochemotherapy. Data were evaluated 6 months after a once-only treatment, indicating that this simple treatment may provide long-term responses in cutaneous metastases, as only three out of 12 calcium electroporated metastases recurred within a year.
During the 1 year of follow–up, six out of seven patients (the six patients with breast cancer) had progression with new cutaneous metastases outside the treatment area indicating only a local treatment effect. The seventh patient, with malignant melanoma, had complete remission in both the treated metastases, but also in untreated metastases, despite no further treatment, indicating a systemic immune response [Citation23]. Four months prior to inclusion the patient had undergone standard electrochemotherapy on a large area on her left limb including more than 100 metastases. A systemic immune response after treatment with electrochemotherapy alone has not been described in patients before.
Adverse events
Only local adverse events were reported and for both treatments the adverse events were minimal. No grade 3 or 4 toxicities were reported, in fact only grade 1 local adverse events were seen after both treatments.
Ulcers appeared in treatment areas after both calcium electroporation and electrochemotherapy, but none of the ulcers extended beyond the tumor margins, indicating that normal skin was spared (). The selectivity is already known in electrochemotherapy, but for calcium electroporation this finding supports data from preclinical in vitro studies [Citation16,Citation26,Citation28].
There was a benefit in favor of calcium electroporation with regards to cosmetic outcome as hyperpigmentation appeared in 26% of the metastases treated with bleomycin and no difference in pigmentation was seen in metastases treated with calcium.
Response in previous irradiated metastases
The percentage of complete responses in metastases treated with calcium electroporation and electrochemotherapy were almost alike (calcium, 66% vs. bleomycin, 68%). On the other hand, irradiation seemed to strongly influence response as 81% of non-irradiated metastases had complete response after 6-months of follow-up, in contrary to previously irradiated metastases being only 46%. A contributing factor is likely that in several of the cases the irradiated skin appeared hard and fibrotic, making it difficult to insert the needles from the electrode and reach the deeper tumor margin. Furthermore, poor distribution of calcium or bleomycin in the hard fibrotic skin is conceivable.
Measured delivered current was significantly lower in irradiated metastases compared to non-irradiated (), which may be due to less conductivity in the fibrotic tissue and challenges with insertion of the needle electrode.
Delivered current
There was no significant difference in measured delivered current in the 2 treatment arms, which may be due to the very small injection volumes relative to the tumor volume. This difference in conductivity may be more relevant in treatment of larger tumors with injection of larger volumes of calcium.
Limitation
The study design required that the patients were followed for 6 months without change of treatment regimen, which made inclusion a challenge with mainly inclusion of breast cancer patients. For these positive results to become firmly established, confirmatory studies are needed for both metastases from breast cancer but also from other histologies.
Perspectives
Calcium electroporation could be considered as a future treatment for small cutaneous metastases, it is a simple treatment and the better cosmetic outcome, compared to electrochemotherapy, is an advantage that not should be underestimated for patients with visible cancer disease. Though calcium cannot be administered intravenously as bleomycin, it is easy to manage as it is not cytotoxic by itself. The perspectives of calcium electroporation also include treatment of larger tumors and internal tumors as new electrodes for internal use are being developed. Finally, calcium electroporation is cheap, compared to many anticancer treatments, which also could be an advantage for low-income countries.
Conclusion
This double-blinded randomized clinical study clearly indicates that calcium electroporation is feasible in clinical settings with minimal toxicity, and is effective in local tumor reduction.
This study showed no significant difference in delivered current in metastases treated with calcium compared to metastases treated with bleomycin.
Disclosure statement
Julie Gehl reports a conflict of interest regarding a pending patent on calcium electroporation (PCT/DK2012/050496).
Additional information
Funding
References
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228–236.
- Campana LG, Mocellin S, Basso M, et al. Bleomycin-based electrochemotherapy: clinical outcome from a single institution's experience with 52 patients. Ann Surg Oncol. 2009;16:191–199.
- Campana LG, Valpione S, Falci C, et al. The activity and safety of electrochemotherapy in persistent chest wall recurrence from breast cancer after mastectomy: a phase-II study. Breast Cancer Res Treat. 2012;134:1169–1178.
- Heller R, Jaroszeski MJ, Reintgen DS, et al. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer. 1998;83:148–157.
- Kunte C, Letule V, Gehl J, et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol 2017;176:1475–1485.
- Larkin JO, Collins CG, Aarons S, et al. Electrochemotherapy: aspects of preclinical development and early clinical experience. Ann Surg. 2007;245:469–479.
- Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy – an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Ejc Supplements. 2006;4:3–13.
- Matthiessen LW, Chalmers RL, Sainsbury DC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50:621–629.
- Mir LM, Glass LF, Sersa G, et al. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br J Cancer. 1998;77:2336–2342.
- Quaglino P, Mortera C, Osella-Abate S, et al. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann Surg Oncol. 2008;15:2215–2222.
- Rodriguez-Cuevas S, Barroso-Bravo S, Almanza-Estrada J, et al. Electrochemotherapy in primary and metastatic skin tumors: phase II trial using intralesional bleomycin. Arch Med Res. 2001;32:273–276.
- Solari N, Spagnolo F, Ponte E, et al. Electrochemotherapy for the management of cutaneous and subcutaneous metastasis: a series of 39 patients treated with palliative intent. J Surg Oncol. 2014;109:270–274.
- Curatolo P, Quaglino P, Marenco F, et al. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Ann Surg Oncol. 2012;19:192–198.
- Campana LG, Valpione S, Mocellin S, et al. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. 2012;99:821–830.
- Frandsen SK, Gissel H, Hojman P, et al. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72:1336–1341.
- Frandsen SK, Kruger MB, Mangalanathan UM, et al. Normal and malignant cells exhibit differential responses to calcium electroporation. Cancer Res. accepted 2017.
- ecorg-acrin.org [internet]. Philadelphia: ECOG-Acrin cancer research group; 2016 [updated 2016; cited 2017 Jun 25]. Available from: ecorg-acrin.org/resources/ecog-performance-status.
- Frandsen SK, Gissel H, Hojman P, et al. Calcium electroporation in three cell lines: a comparison of bleomycin and calcium, calcium compounds, and pulsing conditions. Biochim Biophys Acta. 2014;1840:1204–1208.
- Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator (TM) by means of invasive or non-invasive electrodes. Ejc Supplements. 2006;4:14–25.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247.
- Common Terminology Criteria for adverse Events (CTCAE) [Internet], Version 4.0. Published May 28, 2009 (v4.03:June 14, 2010). [cited 2017 July 5]. Washington, D.C.: U.S. Department of Health and Human Services, National Cancer Institute. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- Quaglino P, Matthiessen LW, Curatolo P, et al. Predicting patients at risk for pain associated with electrochemotherapy. Acta Oncol. 2015;54(3):298–306.
- Falk H, Lambaa S, Johannessen HH, et al. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma – a case report. Acta Oncol. 2017;56(8):1126–1131.
- National Institute for Health and Care Excellence (NICE). Electrochemotherapy for metastases in the skin from tumours of non-skin origin. IPG446. London, UK: NICE; 2013.
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529.
- Frandsen SK, Gibot L, Madi M, et al. Calcium electroporation: evidence for differential effects in normal and malignant cell lines, evaluated in a 3D spheroid model. PLoS ONE. 2015;10:e0144028.
- Hansen EL, Sozer EB, Romeo S, et al. Dose-dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength. PLoS ONE. 2015;10:e0122973.
- Zielichowska A, Daczewska M, Saczko J, et al. Applications of calcium electroporation to effective apoptosis induction in fibrosarcoma cells and stimulation of normal muscle cells. Bioelectrochemistry. 2016;109:70–78.
