Abstract
Background: The incidence of vulvar cancer in Germany is increasing. Moreover, gynaecological oncologists reported observing increasing numbers of women presenting with small tumours. The aim of the present study is to validate this observation on a population level and to extend available incidence data.
Material and methods: Data from the population-based Saarland Cancer Registry were used and included 1136 women diagnosed with invasive vulvar cancer (ICD-9 codes: 181.1-181.4, ICD-10 code: C51) between 1974 and 2013. Multiple imputation methodology was used to overcome loss of precision and potential bias resulting from incomplete data. Incidence trends were investigated with regard to age at diagnosis, tumour size and clinical stage, morphology and histopathologic grade.
Results: The age-standardised incidence rate of vulvar cancer increased from 1.6 cases per 100,000 women per year in 1974–78 to 7.9 in 2009–13, representing an increase across all age groups. Since 1989–93, an almost exclusive increase in the incidence of small tumours ≤2 cm in the greatest dimension from 1.2 to 6.6 and of squamous cell carcinomas from 1.7 to 7.1 was observed, whereas the number of larger tumours and other invasive cancers remained rather constant. Patients aged ≥75 years generally suffered from more advanced tumours at the time of diagnosis.
Conclusions: An increase in vulvar cancer incidence of a size as observed in this study has not been reported thus far for any other European region. Furthermore, the analyses confirmed the observation of increasing numbers of women presenting with small tumours. The results of the age-specific analyses point to both human papillomavirus infection and non-infectious factors as explanations for the observed increase in squamous cell carcinomas.
Background
Invasive malignant tumours of the vulva (vulvar cancer) are rather rare types of cancer that predominantly affect elderly women and represent about 5% of the female genital tract malignancies [Citation1]. A number of risk factors have been identified and include age, high-risk oncogenic human papillomavirus (HPV) infection that is responsible for up to half of all vulvar cancers mainly affecting women below age 65 [Citation1], smoking [Citation2] or immunosuppression and human immunodeficiency virus (HIV) infection [Citation3]. Vulvar intraepithelial neoplasia (VIN) as precancerous changes [Citation4] may be related to HPV infection [Citation5] or could be based on chronic skin conditions such as Lichen sclerosus [Citation6].
Almost all malignant tumours of the vulva are squamous cell carcinomas, which represent about 80–95% of all malignant vulvar cancers [Citation1]. Squamous cell carcinomas can be categorised into two groups: keratinising squamous cell carcinomas with little association with HPV infection and warty and basaloid squamous cell carcinomas with a strong association with HPV infection [Citation1]. The remaining malignant tumours represent malignant melanomas, Paget disease, basal cell carcinomas, sarcomas and other morphologic types [Citation7,Citation8].
Prior to the new millennium, the incidence of vulvar cancer in Germany had been quite similar to those in other European regions or industrialised countries [Citation9,Citation10]. In recent years, however, a significant increase in the incidence of vulvar cancer has been observed in some German regions [Citation11], resulting in a much higher incidence in Germany compared to many neighbouring countries [Citation12,Citation13]. Along with the increase in incidence in Germany, a moderate increase in vulvar cancer mortality has been observed [Citation13]. Gynaecological oncologists reported observing increasing numbers of women presenting with small tumours.
This study aims to further investigate the aforementioned observation, to extend available data on the incidence of vulvar cancer in Germany and to provide explanations for the observed increase in vulvar cancer incidence with regard to known risk factors and public health measures.
Material and methods
Cancer data from the population-based Saarland Cancer Registry (CR) were used for this study. In the federal state of Saarland situated in southwest Germany with a population of 1.02 million inhabitants in 2010, cancer reporting is mandatory by law. The CR obtains notifications from hospitals, outpatient clinics, radiotherapy departments, pathology laboratories and doctors in private practice. Furthermore, the CR obtains death certificates of each death from local health authorities.
For the analyses, data from 1136 women diagnosed with a first invasive vulvar cancer between 1974 and 2013 were used (codes of the International Classification of Diseases according to ninth (ICD-9) and 10th revision (ICD-10): 184.1-184.4 and C51 [Citation14,Citation15]). The data included information on date of diagnosis, age at diagnosis, basis of diagnosis, morphology (according to the third edition of the International Classification of Diseases for Oncology (ICD-O-3) [Citation16]), histopathologic grade, tumour size and extent of disease (no metastases, involvement of regional lymph nodes and involvement of distant sites and organs) according to the TNM classification (based on editions 4 and 6 used from 1989 and 2003 onward, respectively) [Citation17,Citation18], as well as the date and cause of death (according to ICD-9 and ICD-10) or the date of the end of follow-up (31 December 2013, at the latest).
While information on socio-demographic items and cancer site was available for all patients, information on tumour morphology, histopathologic grade, tumour size and extent of disease had been collected since the mid 1980s but was partially missing. Overall, data on these items were available for 97%, 69%, 69% and 48% of the tumours diagnosed after 1989, respectively. To overcome the loss of precision and potential bias resulting from the exclusion of incomplete observations [Citation19,Citation20], multiple imputation (MI) by chained equations was used to derive replacement values for the missing values [Citation21]. Overall, 15 multiply imputed datasets were derived and used for descriptive analyses and incidence estimation. Details on the statistical model used for MI, data sampling and a combination of the estimates derived for each sampled dataset may be found in the provided supplementary materials.
Trends of age-standardised rates (ASR) and truncated age-standardised rates (TASR) of vulvar cancer incidence and mortality were derived for subsequent calendar intervals of 5 years between 1974 and 2013. The ASR and TASR of incidence by tumour size, clinical stage, morphology and histopathologic grade were derived for the calendar period 1989–2013. The Europe standard population was used for standardisation.
Descriptive analyses with regard to age at diagnosis (≤54, 55–74 and ≥75 years), the size of the primary tumour (T1, T2 or T3/4), clinical stage (I, II, III or IV according to the International Federation of Gynecology and Obstetrics (FIGO) based on the sixth edition of the TNM) [Citation22], tumour morphology (squamous cell carcinoma or other invasive cancers; the respective ICD-O-3 morphology codes are provided with ) and histopathologic grade (G1, G2 or G3/4) were performed for subsequent calendar intervals of 5 years. and show results from the calendar periods 1989–93, 1999–2003 and 2009–13, respectively. Along with these results, p values of chi-squared tests (for homogeneity) for differences in patient and tumour characteristics over time or between age groups and Mood’s tests for trends in the median age at diagnosis were provided.
Table 1. Characteristics of patients with invasive vulvar cancer (ICD-9: 184.1-184.4, ICD-10: C51) from Saarland diagnosed from 1989–1993, 1999–2003 and 2009–2013.
Table 2. Truncated age-standardised incidence rates of invasive vulvar cancer (ICD-9: 184.1–184.4, ICD-10: C51) according to tumour size, clinical stage, tumour morphology and histopathologic grade from Saarland in 1989–1993, 1999–2003 and 2009–2013 and distribution of tumour characteristics of patients diagnosed in 2009–2013 across age groups.
The supplementary materials also include the results of descriptive analyses after exclusion of observations with missing values (Supplementary Table 1) and the results of descriptive analyses and ASR and TASR of vulvar cancer incidence derived from the multiply imputed data presenting data from all calendar intervals and detailed analyses by tumour morphology (Supplementary Tables 2 and 3).
For data preparation, model estimation, sampling and statistical analyses, the R language and environment for statistical computing (version 3.1.3) was used [Citation23].
Results
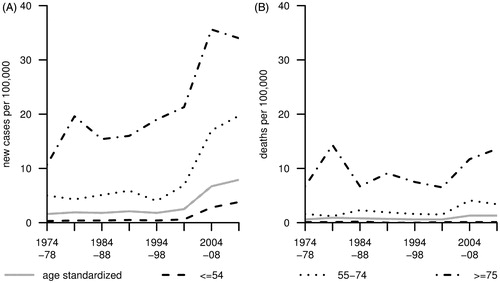
presents trends of ASR and TASR of incidence and mortality from invasive vulvar cancer. The ASR of incidence has increased by about 390% from 1.6 cases per 100,000 women per year in 1974–78 to 7.9 in 2009–13 (unless otherwise stated, the ASR and TASR are given in the remaining text). Over that calendar period, an increase in incidence from 0.3 to 3.8 (+1160%), 5.0 to 19.7 (+290%) and 10.7 to 34.0 (+220%) was observed for women ≤54, 55–74 and ≥75 years, respectively (). In women up to age 74, the observed increase in incidence essentially occurred over the past 15 years. Over the study period, the ASR of mortality increased by about 120% from 0.6 to 1.3 deaths per 100,000 women per year. The TASR remained at 0.1 and increased from 1.6 to 3.4 (+110%) and 6.7 to 13.6 (+105%), respectively ().
Figure 1. Age-standardised incidence (A) and mortality (B) of invasive vulvar cancer (ICD-9: 184.1-184.4, ICD-10: C51) in Saarland between 1974 and 2013 (truncated age-standardised rates are given for the presented age classes).
presents numbers and tumour characteristics of patients diagnosed 1989–93, 1999–2003 and 2009–13. Over time, the numbers of registered cases increased from 91 in 1989–93 to 325 in 2009–13. The median age at diagnosis dropped from 72 to 69 years. During the study period, the proportions of microscopically verified tumours and fatal cancers with a death certificate only notification were above 95% and ranged between 2% and 4%, respectively (Supplementary Table 1).
Between 1989 and 2013, an almost exclusive increase in small tumours ≤2 cm in the greatest dimension (T1) was observed. Here, the number of diagnosed tumours increased from 31 in 1989–93 to 257 in 2009–13 (+730%; representing 34% and 79% of all tumours), whereas the number of tumours sized >2 cm or spreading to adjacent structures (T2–4) remained rather constant (60 and 68; +15%). Accordingly, the proportion of clinical stage I cancers increased over time from 31% to 52%, whereas the proportion of metastasised stage IV cancers decreased from 21% in 1989–93 to 10% in 2009–13, respectively. The number of squamous cell carcinomas increased from 74 in 1989–93 to 287 in 2009–13 (+275%), whereas the number of all remaining cancer types combined (including basal cell carcinoma, adenocarcinoma, Paget disease, malignant melanoma and other/unspecified tumours) remained almost constant over time (17 and 38, +105%). Simultaneously, the proportion of lesions with intermediate (G2) or poor (G3/4) differentiation increased from 83% to 93%, respectively.
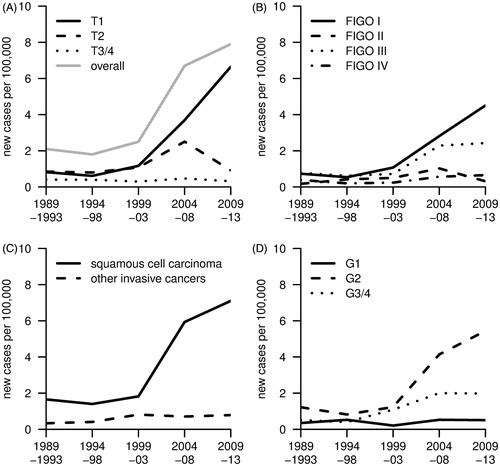
depicts the development of the ASR of incidence of vulvar carcinoma between 1989 and 2013 by tumour size, clinical stage, morphology and histopathologic grade. The charts well reflect the data presented in . The ASR of T1 tumours increased from 1.2 cases per 100,000 women per year in 1999–2003 to 6.6 in 2009–13. A transient increase in the incidence of tumours >2 cm in the greatest dimension but confined to the vulva (T2) to 2.5 in 2004–08 was observed, after 1.1 in 1999–2003 and 0.9 in 2009–13, respectively (). Along with the increase in T1 tumours, the incidence of clinical stage I and III tumours increased from 1.1 and 0.7 in 1999–2003 to 4.5 and 2.4 in the most recent calendar period, respectively. The incidence of clinical stage II and IV tumours remained rather stable below 1.0 and 0.7 new cases per 100,000 women per year, respectively (). The ASR of incidence of squamous cell carcinomas increased from 1.7 to 7.1 (+320%), which essentially occurred between 1999 and 2013 and almost entirely accounted for the observed increase in incidence (). At the same time, the ASR of incidence of tumours with intermediate and poor differentiation (G2 and G3/4) increased from 1.2 and 0.5 to 5.4 and 2.0, respectively, whereas the incidence of well-differentiated tumours (G1) remained unchanged ().
Figure 2. Age-standardised incidence of invasive vulvar carcinoma (ICD-9: 184.1-184.4, ICD-10: C51) in Saarland between 1989 and 2013 by tumour size (A), clinical stage (B), tumour morphology (C) and histopathologic grade (D).
presents trends of the TASR of vulvar cancer incidence according to tumour characteristics and the distribution of tumour characteristics in 2009–13 by age. The observed increases in the TASR of T1 and clinical stage I tumours were comparable and ranged between 5.9-fold and 7.8-fold increase in T1 tumours and 3.9-fold and 6.2-fold increase in clinical stage I tumours. Overall, the proportions of T1 and clinical stage I tumours decreased with age at diagnosis and were 92% and 66% among patients ≤54 years but 68% and 44% among patients ≥75 years in 2009–13, respectively; p values <.001 and .141, respectively. According to the different departure levels in 1989–93, the increase of the TASR of squamous cell carcinomas ranged between 10.3-fold in women ≤54 years and 1.2-fold in women ≥75 years. The distribution of morphologic types and histopathologic grade in 2009–13 was comparable across all age groups. The proportions of squamous cell carcinomas and tumours with intermediate differentiation (G2) ranged between 84% and 92% and 61% and 69%, respectively (p values .375 and 1.000, respectively).
A comparison of the results derived from the multiply imputed data presented in and the results from a conventional analysis excluding observations with missing values (Supplementary Table 1) showed comparable results.
Supplementary Figure 1 presents the development of the ASR of other tumours of the female genital organs and other cancer types associated with HPV infection. Between 1974–79 and 2009–13, the ASR of the incidence of cervical cancer decreased from 24.0 to 12.5, whereas the incidence of ovarian cancer remained rather stable and ranged between 13.6 and 16.4 (Supplementary Figure 1(A)). The ASR of the incidence of malignant tumours of the base of the tongue and the tonsils as well as anal cancer increased during the study period from 0.6 and 0.3 to 1.4 and 2.3, respectively (Supplementary Figure 1(B)).
Discussion
This study based on CR data aimed to further investigate a recently reported increase in the incidence of invasive vulvar cancer in Germany [Citation12] by providing stratified incidence trends with regard to age at diagnosis, tumour size and clinical stage, tumour morphology and histopathologic grade over a period of 25 years or longer. Since the beginning of the millennium, the ASR of the incidence of invasive vulvar cancer tripled from 2.5 to 7.9 cases per 100,000 women per year, which almost entirely resulted from additional squamous cell carcinomas and small T1 tumours. Accordingly, the proportion of T1 tumours increased from 42% to 79%; however, the proportion of small and early stage tumours remained significantly lower among patients aged ≥75 years.
The presented findings are in line with clinical observations from Germany on both an increasing number of vulvar cancer patients and a decreasing age at diagnosis [Citation24]. Furthermore, the study validated the perception of gynaecological oncologists observing an increasing number of patients presenting with small tumours.
A recent study reported an increase in vulvar cancer incidence in Germany from approximately two to four new cases per 100,000 women per year between 1999 and 2011 [Citation12]. As some regions with a completeness of case ascertainment of less than 90% had been included in that study [Citation25], it is very likely that the above-cited incidence figures underestimate the true cancer burden. Therefore, the aforementioned study additionally analysed the development of the vulvar cancer incidence in the federal states of Hamburg and Saarland with longstanding population-based CRs that showed a substantial increase in incidence to about seven new cases per 100,000 women per year in 2011.
Compared to Germany, the incidence of invasive vulvar cancer was lower both in neighbouring countries and in other European or developed countries across the world [Citation11]. In Austria, the Czech Republic, Belgium and the Netherlands, the ASR of incidence ranged between two and three in 2010/2011 [Citation12]. The increase in vulvar cancer incidence observed in some other European countries or in North America was of lower dimensions. The ASR increased from two to three cases per 100,000 women per year between 2001 and 2011 in the Netherlands [Citation12] and from three to four between 1992 and 2008 in Canada [Citation26]. Overall, between 1993 and 2007, no consistent trend in vulvar cancer incidence could be observed across the European regions [Citation9,Citation11]. A study from the Netherlands reported an increase in the incidence of squamous cell carcinomas after 2002 among women below age 60; however, the study did not observe substantial changes in the stage of disease [Citation27].
To explain the increase in cancer burden observed in Germany, possible changes in the prevalence of risk factors of vulvar cancer in the past require consideration. This study revealed a 16-fold increase in vulvar cancer incidence in women below age 54 since 1974 and that squamous cell carcinomas represent virtually all of these additionally diagnosed cancers. This points to an increased incidence of HPV infections in the past, for example, due to a more permissive sexual behaviour in women born in 1940 and after [Citation28]. The observed increases in the incidence of malignant tumours of the base of the tongue and the tonsils as well as anal cancer among women during the study period support this assumption, as these cancers are likewise related to HPV infection [Citation1]. By contrast, prevalence studies have shown rather similar proportions of HPV-infected women in Germany and other northern, western and southern European populations [Citation29], which would expect a comparable burden of vulvar cancers in Germany and other European countries. The observed 3.2-fold increase in vulvar cancer incidence in women aged ≥75 years since 1974 points to a possible contribution of additional risk factors. Here, an increase in chronic skin conditions such as Lichen sclerosus or chronic inflammation and so-called ‘differentiated type’ VIN [Citation6] as well as increasing cigarette smoking among women born after 1930 may have contributed to the observed rise [Citation1,Citation2]. Other established risk factors such as HIV infection or immunosuppression certainly played a very limited role [Citation3,Citation30]. However, based on the available data, a definitive explanation for the observed trends over time and the differences in the incidence between Germany and other European countries may not be elucidated.
As opportunistic screening, the Pap smear test and the clinical examination of the outer and inner female genitals and the breasts are offered annually to women age 20 years or older by statutory health insurance in Germany since 1971 [Citation31]. The annual proportion of women utilising such cancer screening increased to about 45% until the end of the 1990s and remained almost constant thereafter [Citation32,Citation33]. Increasing usage of screening therefore seems unlikely as an explanation for the increasing numbers of vulvar cancers observed during the past 15 years. The observed trends of the ASR of the incidence of cervical and ovarian cancer in the study period support this reasoning.
Increasing awareness of clinicians and increasing utilisation of biopsies in recent years may have led to the detection of additional vulvar lesions. However, it seems implausible that large numbers of invasive vulvar cancers had been missed in the past or that the observed increase in vulvar cancers resulted from overdiagnosis of invasive vulvar carcinomas.
The study findings further indicate that the offered screening examinations of the female genitals were effective as the additional cancers were essentially diagnosed at an early stage. However, some of these tumours already must have metastasised to groin lymph nodes, as the number of clinical stage I tumours did not increase the same amount as did the number of T1 tumours. The overall proportions of patients with metastatic groin lymph nodes ranged between 30% and 38% and was comparable to other findings [Citation34].
A number of important strengths and limitations require careful consideration. For this study, data from the longstanding population-based Saarland CR were used. The CR has been providing high-quality data on cancer incidence and mortality for almost 50 years with an estimated proportion of cancer cases notified at lifetime of at least 90% since the end of the 1990s [Citation13,Citation35]. The CR regularly contributes data to national and international collaborative studies (e.g. [Citation11,Citation25,Citation36]). Even though Saarland constituted only 1.2% of the national population in 2013, it is well representative for Germany as it consists of both urban and rural areas and its health care system. Comparative analyses revealed that cancer incidence and mortality rates of women in Saarland were quite similar to the estimated rates for the entire Germany (e.g. [Citation13,Citation25,Citation35]).
The use of MI allowed to making the best use of the available data. By providing plausible replacement values for missing data, observations with incomplete information can be utilised while preserving key features of the existing data. The sampled values represent the uncertainty about the missing values and allow the derivation of valid statistical inference [Citation37]. The incorporation of observed follow-up time, vital status at the end of follow up and cause of death into the MI model improved its validity as these variables highly correlate with lacking tumour information. Despite the usage of rather broad calendar intervals, the small case numbers limited the degree of detail of the analyses and were responsible for remaining random variation.
Possible artefacts from registration include misclassification issues. To rule out possible overestimation of vulvar cancer incidence, for example as a result of homonym errors due to missed linkage of multiple notifications or misclassification of other malignancies of the female genital organs, the notifications of vulvar malignancies of the calendar years 1993, 2003 and 2011–13 underwent clerical review resulting in a clearance of three out of 249 cases, which did not change the results in any meaningful way.
The observed increase in the incidence of tumours with metastatic regional lymph nodes (clinical stage III) very likely reflects stage migration [Citation38]. This may have resulted from changes in the recommendations concerning resection of groin lymph nodes and an improved sensitivity of staging procedures and technologies over time (e.g. recommendation for staging of the groins in tumours staged T1b [Citation39], sentinel node dissection in patients with early stage vulvar cancer [Citation40,Citation41], thorough examination of dissected lymph nodes [Citation42]), and the advances in the TNM classification [Citation17,Citation18]. In the available data, the TNM data were based on the fourth edition of the classification until 2002 and the sixth edition from 2003 on.
The available information on tumour morphology hampered a categorisation of the squamous cell carcinomas into tumours strongly associated with HPV infection and tumours predominantly associated with non-infectious factors and chronic skin conditions as a substantial proportion of pathology reports included unspecific information on the morphologic characterisation only. In 2009–13, the Saarland CR obtained copies of pathology reports of 96% of the registered squamous cell carcinomas, and the morphologic types were distributed as follows: ‘keratinising’ 68%, ‘not otherwise specified (NOS)’ 23%, ‘nonkeratinising’ 7% and ‘verrucous’ 3%, respectively. Given such a skewed distribution, the MI model used could not be extended any further. For this reason, we used trends of age-specific incidence to assess the contribution of HPV infections and non-infectious factors on the observed incidence trends. To strengthen these conclusions, we additionally utilised data on the incidence trends of other HPV-related cancers.
To summarise, this study extended a previous study which reported an increase in vulvar cancer incidence in Germany. The detailed analyses revealed that the observed increase in tumours in the past 15 years mainly resulted from newly diagnosed squamous cell carcinomas and small tumours. To the best of our knowledge, an increase in vulvar cancer incidence of a dimension as observed for Germany in this analysis has not been observed for any other European country thus far. Therefore, differences in the prevalence of aetiologic factors must be considered.
Further research is required to monitor the geographical differences and future trends of vulvar cancer incidence across German and European regions. Corresponding studies should ideally incorporate not only incident cancers, but also the prevalence of risk factors, and data collection and coding practices in different populations. It may be expected that HPV vaccination may prevent the occurrence of up to two-thirds of intraepithelial lesions and up to half of the carcinomas of the vulva in younger women in the future [Citation43]. Here, population-based CRs are pivotal as they contribute essentially to the quantification of the public health impact of such a measure.
IONC_A_1360513_Supplementary_Information.zip
Download Zip (75.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Stewart BW, Wild CP, editors. World cancer report 2014. Geneva: World Health Organization; 2014.
- Madeleine MM, Daling JR, Carter JJ, et al. Cofactors with human papillomavirus in a population-based study of vulvar cancer. J Natl Cancer Inst. 1997;89:1516–1523.
- Ferenczy A, Coutlee F, Franco E, et al. Human papillomavirus and HIV coinfection and the risk of neoplasias of the lower genital tract: a review of recent developments. Can Med Assoc J. 2003;169:431–434.
- Reyes MC, Cooper K. An update on vulvar intraepithelial neoplasia: terminology and a practical approach to diagnosis. J Clin Pathol. 2014;67:290–294.
- De Vuyst H, Clifford GM, Nascimento MC, et al. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124:1626–1636.
- van de Nieuwenhof HP, Massuger LF, van der Avoort IA, et al. Vulvar squamous cell carcinoma development after diagnosis of VIN increases with age. Eur J Cancer. 2009;45:851–856.
- Hacker NF, Eifel PJ, van der Velden J. Cancer of the vulva. Int J Gynaecol Obstet. 2015;131(Suppl 2):S76–S83.
- Alkatout I, Schubert M, Garbrecht N, et al. Vulvar cancer: epidemiology, clinical presentation, and management options. Int J Womens Health. 2015;7:305–313.
- Parkin DM, Whelan SL, Ferlay J, et al., editors. Cancer incidence in five continents. Volume VIII. IARC Scientific Publications No. 155. Lyon: International Agency for Research on Cancer; 2002.
- Curado MP, Edwards B, Shin HR, et al., editors. Cancer incidence in five continents. Volume IX. IARC Scientific Publications No. 160. Lyon: International Agency for Research on Cancer; 2007.
- Forman D, Bray F, Brewster DH, et al., editors. Cancer incidence in five continents. Volume X. IARC Scientific Publication No. 164. Lyon: International Agency for Research on Cancer; 2014.
- Buttmann-Schweiger N, Klug SJ, Luyten A, et al. Incidence patterns and temporal trends of invasive nonmelanotic vulvar tumors in Germany 1999–2011. A population-based cancer registry analysis. PLoS One. 2015;10:e0128073.
- Robert Koch Institute, Association of Population-based Cancer Registries in Germany, editors. Cancer in Germany 2011/2012. 10th ed. Berlin: Robert Koch Institute; 2015.
- World Health Organization, editor. International classification of diseases. 9th revision. Geneva: World Health Organization; 1977.
- World Health Organization, editor. International statistical classification of diseases and related health problems. 10th revision. Geneva: World Health Organization; 1992.
- Fritz A, Percy C, Jack A, et al., editors. International classification of diseases for oncology (ICD-O). 3rd ed. Geneva: World Health Organization; 2000.
- Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6th ed. New York: Springer; 2002.
- Beahrs OH, Henson DE, Hutter RVP, editors. Manual for staging of cancer. 3rd ed. American Joint Committee on Cancer. New York: Lippincott Company; 1988.
- White IR, Carlin JB. Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Statist Med. 2010;29:2920–2931.
- Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. Hoboken (NJ): John Wiley and Sons; 2002.
- Nur U, Shack LG, Rachet B, et al. Modelling relative survival in the presence of incomplete data: a tutorial. Int J Epidemiol. 2009;39:118–128.
- Beller U, Quinn MA, Benedet JL, et al. Carcinoma of the vulva. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S7–S27.
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2014.
- Hampl M, Deckers-Figiel S, Hampl JA, et al. New aspects of vulvar cancer: changes in localization and age of onset. Gynecol Oncol. 2008;109:340–345.
- Robert Koch Institute, Association of Population-based Cancer Registries in Germany, editors. Cancer in Germany 2009/2010. 9th ed. Berlin: Robert Koch Institute; 2013.
- Akhtar-Danesh N, Elit L, Lytwyn A. Trends in incidence and survival of women with invasive vulvar cancer in the United States and Canada: a population-based study. Gynecol Oncol. 2014;134:314–318.
- Schuurman MS, van den Einden LC, Massuger LF, et al. Trends in incidence and survival of Dutch women with vulvar squamous cell carcinoma. Eur J Cancer. 2013;49:3872–3880.
- Sigusch V. On cultural transformations of sexuality and gender in recent decades. Ger Med Sci. 2004;2:Doc07.
- Bruni L, Diaz M, Castellsague X, et al. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–1799.
- European Centre for Disease Prevention and Control, WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2014. Stockholm: ECDC, 2015.
- Becker N. Epidemiological aspects of cancer screening in Germany. J Cancer Res Clin Oncol. 2003;129:691–702.
- The Federal Health Monitoring System. Chart 3.2.7: Utilization of statutory measures for the early detection of cancer (in Percent; starting from 1991) Bonn: Federal Statistical Office; 2016 [cited 2016 November 21]. Available from: http://www.gbe-bund.de/gbe10/ergebnisse.prc_tab?fid =10607&suchstring=&query_id=&sprache=D&fund_typ=GRA&methode=&vt=&verwandte =1&page_ret =0&seite =1&p_lfd_nr =10&p_news=&p_sprachkz=D&p_uid=gast&p_aid =7554746&hlp_nr =1&p_janein=J.
- Saarland Health Monitoring System. [Utilization of statutory measures for the early detection of cancer. Classification: years, Saarland, age, sex (starting from 1995)] Saarbrücken: Ministry of Social Affairs, Public Health, Women and Family Affairs; 2016 [cited 2016 November 21]. Available from: http://www.gbe.saarland.de/medien/download/07_016_2014.htm.
- Hauspy J, Beiner M, Harley I, et al. Sentinel lymph node in vulvar cancer. Cancer. 2007;110:1015–1023.
- Batzler WU, Hundsdörfer G, Schön D, et al., editors. Krebs in Deutschland. Dritte erweiterte, aktualisierte Ausgabe. Saarbrücken: Arbeitsgemeinschaft Bevölkerungsbezogener Krebsregister in Deutschland; 2002.
- Sant M, Chirlaque Lopez MD, Agresti R, et al. Survival of women with cancers of breast and genital organs in Europe 1999–2007: results of the EUROCARE-5 study. Eur J Cancer. 2015;51:2191–2205.
- Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley and Sons; 1987.
- Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604–1608.
- Homesley HD, Bundy BN, Sedlis A, et al. Prognostic factors for groin node metastasis in squamous cell carcinoma of the vulva (a Gynecologic Oncology Group study). Gynecol Oncol. 1993;49:279–283.
- De Cicco C, Sideri M, Bartolomei M, et al. Sentinel node biopsy in early vulvar cancer. Br J Cancer. 2000;82:295–299.
- Van der Zee AG, Oonk MH, De Hullu JA, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884–889.
- Woelber L, Trillsch F, Kock L, et al. Management of patients with vulvar cancer: a perspective review according to tumour stage. Ther Adv Med Oncol. 2013;5:183–192.
- Hampl M, Sarajuuri H, Wentzensen N, et al. Effect of human papillomavirus vaccines on vulvar, vaginal, and anal intraepithelial lesions and vulvar cancer. Obstet Gynecol. 2006;108:1361–1368.
- Li K-H, Meng X-L, Raghunathan TE, et al. Significance levels from repeated P-values with multiply-imputed data. Stat Sin. 1991;1:65–92.
