Introduction
We have recently demonstrated that the local failure rate among early-stage node-negative breast cancer patients receiving no adjuvant systemic treatment was high among young patients (≤45 years) receiving breast conserving therapy (BCT) [Citation1–3]. Furthermore, this outcome was associated with an increased risk of getting subsequent disseminated disease and cancer-related death [Citation1]. Despite detailed analysis of the possible underlying causes of this age-related prognosis, we were unable to find any association with classical clinico-pathological parameters, or with approximated intrinsic subtypes [Citation4]. Therefore, we sought to further explore the nature of the local recurrences after BCT. Two distinct types of local recurrence have been described, namely ‘true recurrence’ which is considered to be regrowth of tumor cells at the tumor bed, and ‘new primary’ which is a de novo cancer developed in a different area in the residual breast tissue [Citation5]. In theory, such new primary tumors should have the same incidence and characteristics as new primary cancers in the contralateral breast, and therefore the latter is used as a reference. Several comparative studies of primary tumors and local recurrences have been performed, but the definition of a true recurrence has been inconsistent [Citation6–9]. To our knowledge, we are the first to perform such a comparison based on the localization of recurrence and intrinsic subtype.
Patients and methods
We identified 114 patients with local-regional recurrence (60 recurrences in the residual breast or chest wall, 14 in the ipsilateral axilla and 40 new primary tumors in the contralateral breast) as described previously [Citation4]. Briefly, we obtained material from 64 of these patients, including 41 cases with local recurrence and 23 cases with contralateral breast cancer. Recurrences to the axilla were not included. Formalin-fixed paraffin embedded tissue blocks were processed in pairs, one block from the primary tumor and the other from either the local recurrence or the contralateral breast cancer. In the study, RNA from each tumor block was extracted and estrogen (ER), progesterone (PR), human epidermal growth factor 2 (HER2) and Ki67 content were estimated as previously described [Citation4]. Intrinsic subtype called ‘RNA intrinsic subtype 4 (RIS4)’ was assessed based on expression of ER, PR, HER2 and Ki67: LumA (ER+/PR+, HER2−, Ki67 low (<14%)), LumB (ER+/PR+, HER2−, Ki67 high), Lum-HER2 + (ER+/PR+, HER2+), HER2 + (ER−, PR−, HER2+) and Basal (ER−, PR−, HER2−).
Furthermore, tumors from both groups, primary tumors and recurrence, were classified according to the researched-based PAM50-gene signature estimated by Nanostring nCounter gene expression assay [Citation10,Citation11].
In brief, unstained 10 µm paraffin section were mounted on positively charged glass microscope slides, baked overnight on a slide warmer set to 45 °C, deparaffinated by CitriSolv (Fisher Cat # 22-143-975), rehydrated with 3% glycerol and macro-dissected. The RNA extraction was performed as described in the instruction of the Roche High Pure RNA Paraffin Kit, analyzed with the RUO PAM50 CodeSet on a Nanostring platform, and then the public PAM50-algorithms were performed as described by Parker et al. [Citation12]. The tumors were classified as either Luminal A, Luminal B, HER2-enriched, Basal-like or Normal-like.
Results and discussion
We defined a local recurrence as a ‘true recurrence’ if it occurred in the same position (i.e. scar) and had a similar intrinsic subtype as the primary tumor. However, a RIS4 subtype change from LumA to LumB was also considered to be a true recurrence, since they differed in the value of Ki67-stained cells only, and this statement can be arbitrary [Citation13]. In the PAM50 analysis, however, we considered Luminal A and B to be different.
The correlation between subtypes of the index (primary) tumor and the local recurrence (LR) or the contralateral breast cancer (CC) is demonstrated in . A total of 23 CC were identified, occurring equally in younger (≤45 years, N = 13) and older patients (>45 years, N = 10), irrespective of the surgical treatment of the primary tumor.
Figure 1. Correlation between subtypes of the index tumor (marked with blue) and contralateral breast cancer (Contra, marked with red) or local recurrence (Local, marked with red). Surgical treatment of the index tumor was either mastectomy (Mastec) or breast conserving surgery + radio therapy (BCT). Local recurrence (Local) was subdivided into three subgroups: if primary treatment was mastectomy, if primary treatment was BCT and recurrence was developed in the scar or if primary treatment was BCT and recurrence was most likely outside the scar. Younger: ≤45 years, older >45 years.
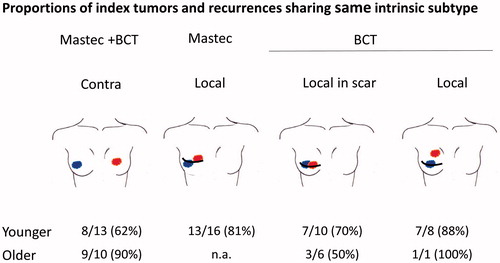
The RIS4 subtype of the CC tumors was classified as: 14 LumA, 3 LumB, 4 Lum-HER2+, 1 HER2+ and 1 Basal, whereas the RIS4 subtypes of the primary index tumors were 20 LumA tumors and 3 LumB tumors (). The biology of these new CC tumors was slightly different in the two age groups. Among the younger patients, the RIS4 subtype of index and CC was different in 5 of 13 cases, whereas it differed only in 1 of 10 cases in the older patients ().
Figure 2. Relationship between intrinsic subtypes of the primary index tumor and local recurrence or contralateral breast cancer. Intrinsic subtype classification was based on either RIS4 (A) or PAM50 (B).
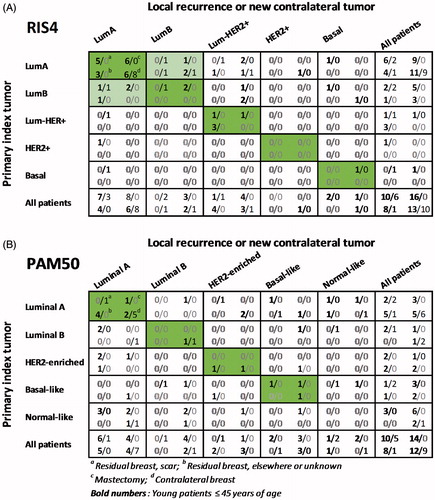
Forty-one patients had a recurrence in the residual breast after BCT or in the chest wall after mastectomy. In the mastectomy group, only material from younger patients with LR was available (N = 16), and the RIS4 subtypes were as follows: index tumor (9 LumA, 5 LumB, 1 Lum-HER2+ and 1 Basal) and LR (8 LumA, 3 LumB, 4 Lum-HER2+, 1 Basal). The LR had subtypes different to that of the index tumor in 3 of 16 cases, which was of the same magnitude as the younger patients with CC.
In the BCT group among the older patients, 6 of 7 LR were located in the scar. The RIS4 subtypes of these were as follows: index tumor (2 LumA, 2 LumB, 1 Lum-HER2+ and 1 Basal) and LR (3 LumA, 2 LumB, 1 Lum-HER2+). The RIS4 subtypes appeared different from the index tumor in 3 of 6 cases.
In the BCT group among the younger patients, 10 of 18 LR were located in the scar. The RIS4 subtypes were as follows: index tumor (6 LumA, 2 LumB, 1 Lum-HER2+ and 1 HER2+) and LR (7 LumA, 1 Lum-HER2+, 2 Basal). The RIS4 subtypes appeared different from the index tumor in 3 of 10 cases. The eight LR with no known location in the original scar had RIS4 subtypes as follows: index tumor (4 LumA, 1 LumB, 3 Lum-HER2+) and LR (4 LumA, 4 Lum-HER2+). In one of eight cases the LR differed to that of the index tumor. Only 7 of 18 LR among the younger patients with BCT would satisfy our definition of a true recurrence. Thus, there is no indication that true recurrences is a dominant feature of younger BCT patients. Furthermore, the few true recurrences observed do not seem to be linked to a higher risk of distant metastasis.
To explore the biological nature of LR further, we performed intrinsic subtyping based on PAM50 analysis, which provided a successful classification in 59 of 64 cases ( and Citation3). It is noteworthy that we successfully could analyze DNA from 123 of 128 specimens, even the FFPE blocks from the index tumor were older than 18 years.
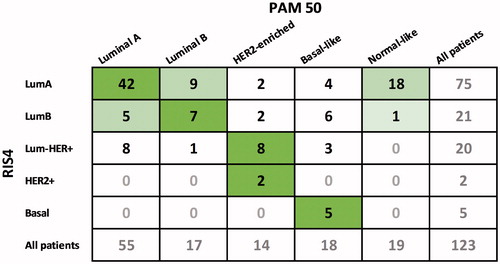
The distribution of intrinsic subtypes based on PAM50 was as follows: index tumor (24 Luminal A, 1 Luminal B, 7 HER2-enriched, 9 Basal-like and 12 normal-like), LR (13 Luminal A, 4 Luminal B, 5 HER2-enriched, 7 Basal-like and 9 normal-like) and CC (11 Luminal A, 3 Luminal B, 2 HER2-enriched, 2 Basal-like, 3 normal-like). In doing so, we observed an overall agreement () with our RIS4 classification in about 75% of the tumors, which seemed highest in patients with HER2-negative tumors. This level of agreement is consistent with findings in other studies [Citation14–17], and it illustrates that intrinsic subtype classification is linked to a significant amount of uncertainty depending on the method used, and consequently, if this nomenclature is used, it should be with some concern. In 19 of the 123 cases, PAM50 classified the tumor material as ‘Normal-like’, when compared with corresponding RIS4 subtype 18 of 19 cases had a LumA subtype.
The PAM50-based intrinsic subtype of the index tumor was compared with LR or CC (). In contrast to the RIS4 subtype comparison, we considered Luminal A and Luminal B to be two distinct different subtypes, since the PAM50 analysis depended on the value of 50 different genes, and therefore was expected to perform a more exact discrimination between the Luminal subtypes [Citation18]. The cases with normal-like tumors were not taken into account, when the pairs of tumors were compared.
Based on the PAM50 analysis, 17 of the 23 CC had the following intrinsic subtype classification: index tumor (10 Luminal A, 3 Luminal B, 2 HER2+ and 2 Basal-like) and CC (9 Luminal A, 2 Luminal B, 3 HER2-enriched and 3 Basal-like). A similar age depending nature as observed with RIS4 subtypes were found; among the younger patients, the index tumor and the CC differed in 5 of the 10 cases, whereas only one of seven differed among the older patients. In the latter case, the index tumor and CC were classified as Luminal B and Luminal A, respectively.
In the mastectomy group, among the younger patients only 7 of 17 LR could be compared with the index tumor, when the PAM50 subtype was applied: Index tumor (2 Luminal A, 1 Luminal B, 1 HER2-enriched and 3 Basal-like) and LR (2 Luminal A, 2 Luminal B and 3 Basal-like). The LR had subtypes different to that of the index tumor in four of seven cases.
In the BCT group among the younger patients, six LR in the scar were classified by PAM50 as follows: index tumor (1 Luminal A, 2 Luminal B, 2 HER2-enriched and 1 Basal-like) and LR (4 Luminal A, and 2 Basal-like). Seven LR with no known location were classified as follows: index tumor (4 Luminal A, 2 HER2-enriched and 1 Basal-like) and LR (5 Luminal A, and 2 HER2-enriched). The index tumor and LR developed in the scar had different PAM50 subtypes in five of six cases, whereas only two of seven differed among the other LR.
Our material was not large enough to perform robust statistical analysis to look deeper into the relationship with time to recurrence and distant metastasis.
In conclusion, the current outcome of a more detailed intrinsic subtype analysis does not seem to provide further insight into the reasons for the nature of the excess frequency of in-breast recurrences in younger low-risk patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Laurberg T, Lyngholm CD, Christiansen P, et al. Long-term age-dependent failure pattern after breast-conserving therapy or mastectomy among Danish lymph-node-negative breast cancer patients. Radiother Oncol. 2016;120:98–106.
- Lyngholm CD, Laurberg T, Alsner J, et al. Failure pattern and survival after breast conserving therapy. Long-term results of the Danish Breast Cancer Group (DBCG) 89TM cohort. Acta Oncol. 2016;55:983–992.
- Bodilsen A, Offersen BV, Christiansen P, et al. Pattern of relapse after breast conserving therapy, a study of 1519 early breast cancer patients treated in the Central Region of Denmark 2000–2009. Acta Oncol. 2016;55:964–969.
- Laurberg T, Alsner J, Tramm T, et al. Impact of age, intrinsic subtype and local treatment on long-term local-regional recurrence and breast cancer mortality among low-risk breast cancer patients. Acta Oncol. 2017;56:59–67.
- Veronesi U, Marubini E, Del VM, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst. 1995;87:19–27.
- Huang E, Buchholz TA, Meric F, et al. Classifying local disease recurrences after breast conservation therapy based on location and histology: new primary tumors have more favorable outcomes than true local disease recurrences. Cancer. 2002;95:2059–2067.
- Gujral DM, Sumo G, Owen JR, et al. Ipsilateral breast tumor relapse: local recurrence versus new primary tumor and the effect of whole-breast radiotherapy on the rate of new primaries. Int J Radiat Oncol Biol Phys. 2011;79:19–25.
- Panet-Raymond V, Truong PT, McDonald RE, et al. True recurrence versus new primary: an analysis of ipsilateral breast tumor recurrences after breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2011;81:409–417.
- Hattangadi-Gluth JA, Wo JY, Nguyen PL, et al. Basal subtype of invasive breast cancer is associated with a higher risk of true recurrence after conventional breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2012;82:1185–1191.
- Fortina P, Surrey S. Digital mRNA profiling. Nat Biotechnol. 2008;26:293–294.
- Jørgensen CLT, Nielsen TO, Bjerre KD, et al. PAM50 breast cancer intrinsic subtypes and effect of gemcitabine in advanced breast cancer patients. Acta Oncol. 2014;53:776–787.
- Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167.
- Polley M-YC, Leung SCY, McShane LM, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105:1897–1906.
- Tramm T, Kyndi M, Myhre S, et al. Relationship between the prognostic and predictive value of the intrinsic subtypes and a validated gene profile predictive of loco-regional control and benefit from post-mastectomy radiotherapy in patients with high-risk breast cancer. Acta Oncol. 2014;53:1337–1346.
- Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–5232.
- Falato C, Tobin NP, Lorent J, et al. Intrinsic subtypes and genomic signatures of primary breast cancer and prognosis after systemic relapse. Mol Oncol. 2016;10:517–525.
- Bartlett JMS, Bayani J, Marshall A, et al. Comparing breast cancer multiparameter tests in the OPTIMA prelim trial: no test is more equal than the others. J Natl Cancer Inst. 2016;108:djw050.
- Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26–S35.