Abstract
Background: For patients with recurrent prostate cancer after radical prostatectomy (RP), salvage radiotherapy (SRT) is a second chance of cure. However, depending on risk factors, 40–70% of the patients experience further progression. With a focus on the pre- and post-SRT serum level of the prostate-specific antigen (PSA), we assessed the determinants of the long-term outcome after SRT.
Patient and methods: Between 1997 and 2011, 464 patients received 3D-conformal SRT with median 66.6 Gy. The median PSA level before SRT was 0.31 ng/ml. In our retrospective analysis, post-SRT progression was defined as either a rising PSA >0.2 ng/ml above the nadir, or the application of anti-androgens or clinical recurrence. A PSA <0.1 ng/ml was termed undetectable. We analyzed the data with the Kaplan–Meier method (Logrank test) and multivariable Cox regression.
Results: The median follow-up was 5.9 years. Overall, 178 patients had recurrence, 13 developed distant metastases and 30 died. Univariate, a pre-RP PSA <10 ng/ml, pathological stage pT <3, Gleason score <8, positive surgical margins, a pre-SRT PSA <0.2 ng/ml and a post-SRT PSA nadir <0.1 ng/ml correlated with fewer and later second recurrences. In a multivariable Cox model, pT, Gleason score, margin status and pre-SRT PSA were significant covariates of progression. If the post-SRT PSA response was included in the regression analysis, then a nadir ≥0.1 ng/ml was the strongest risk factor. Initiating SRT at a PSA <0.2 ng/ml correlated with a post-SRT PSA <0.1 ng/ml. Men who achieved an undetectable post-SRT PSA nadir also had lower rates of metastases and a better overall survival. However, there were too few events for Cox regression analysis of these two endpoints.
Conclusions: Early SRT at a PSA <0.2 ng/ml correlates with re-achieving an undetectable PSA, which predicts improved freedom from progression and metastases and better overall survival.
Introduction
The first-line treatment options for prostate cancer (PCa) are radical prostatectomy (RP) or definitive radiotherapy (RT), the latter combined with androgen deprivation (ADT) where indicated. So far, there is no high-level evidence, which approach yields the better outcome. If RP is chosen, then the best results are achieved with locally confined disease [Citation1]. However, depending on risk factors such as an advanced pathological stage (pT3-4), a high Gleason score (GLS 8–10) or positive surgical margins (R1), 50–80% of the patients experience recurrence after RP [Citation2]. In a study with over 12,000 patients, the preoperative nomogram predictions of 5-year prostate-specific antigen (PSA) progression-free probability correlated with the observed 15-year prostate cancer-specific mortality (PCSM): patients with a 1–25% risk of biochemical recurrences had 5% PCSM, those with a 76–99% risk had 38% PCSM [Citation3]. In a cohort of 450 men who never received adjuvant or salvage therapy, metastasis-free survival after post-RP biochemical relapse decreased by 25% every five years [Citation4].
For men with PSA recurrence, salvage radiotherapy (SRT) offers a second chance of cure. Overall, around 60% re-achieve an undetectable PSA after SRT, and 80% of these patients are free from progression, 5 years after SRT [Citation5,Citation6]. However, 40–70% of the SRT patients suffer a second relapse. The rate of these recurrences depends on pre-SRT parameters with the PSA as a pivotal factor. Guidelines differ in their recommendations, when to state biochemical recurrence and initiate SRT. However, a rising PSA >0.2 ng/ml is a widely accepted criterion to state PSA relapse and there is consensus that SRT should be given early [Citation7]. While ‘early’ can be up to 0.5 ng/ml, levels down to 0.03 ng/ml have been suggested for specific settings in order not to compromise the efficacy of local control [Citation8,Citation9]. Recently, a retrospective study on 1106 PCa patients with a median follow-up of 8.9 years, reported on post-RP SRT (1987–2013) at a PSA ≤0.5 ng/ml compared with later treatment: biochemical progression, metastasis and tumor mortality were significantly reduced with the earlier intervention. The advantage was also observed for overall survival, however with p = .14. Besides the pre-SRT PSA, tumor stage and GLS were associated with all four endpoints, while the post-SRT PSA was not analyzed as a potentially independent variable [Citation10].
In fact, the post-SRT PSA appears to be a prognostic marker [Citation11–13]. A decline below 0.1 ng/ml can be regarded as a first favorable treatment result. Failing such a response may identify SRT patients who will require an additional therapy at some time. Here, we report the long-term outcome of PCa patients who received SRT with comparably recent techniques. We focus on the pre-SRT PSA at cutoff 0.2 ng/ml and on the PSA response after SRT, the latter not only as an endpoint, but also as a prognostic parameter.
Material and methods
In this retrospective analysis, we included 464 PCa patients of two university centers, who had RP between 1989 and 2011. All were stated node negative, 448 pN0 and 16 cN0 (lymphadenectomy not indicated). Forty-six patients (10%) received pre-RP ADT. Cases with ADT between RP and SRT were excluded. summarizes the baseline patient characteristics.
Table 1. Baseline characteristics of 464 prostate cancer patients receiving salvage radiotherapy (SRT) for postprostatectomy recurrence.
After recurrence, the patients received SRT (1997–2011) with median 66.6 (range 59.4–72) Gy. All had 3D-conformal treatment, including 13% with intensity-modulated radiotherapy (IMRT). The treatment volume covered the prostate bed (plus the bed of seminal vesicles for pT3-4) with 1 cm security margins.
We report on a median follow-up of 5.9 years (max 14.4 years). Follow-up information was in part obtained through inquiry from the attending practitioners. Due to the incoherent follow-up sequences, we did not calculate kinetic PSA parameters. The major endpoint was post-SRT progression according to Stephenson’s criteria: a rising PSA 0.2 ng/ml above the nadir or the initiation of ADT or clinical progression [Citation5]. As laboratory standards varied during the reported period, we defined a PSA <0.1 ng/ml as undetectable. Distant metastases and overall survival (OS) were additional endpoints. There were too few confirmed events for a reasonable analysis of PCSM. We used WinStat and SPSS for descriptive statistics, Kaplan–Meier analysis (log-rank test) and multivariable Cox regression.
All information was collected from clinical patient files, updated in part with information obtained from patients’ local practitioners or urologists. Approval for the study was obtained from the Ulm University Ethics Committee (391/15).
Results
After prostatectomy, 358 patients (77%) re-achieved an undetectable PSA, 104 retained a PSA >0.1 ng/ml and for two patients, only the pre-SRT PSA was available. The median time from RP to first recurrence was 11 (IQR 4.3–26.6) months and from recurrence to SRT 7.6 (IQR 2.3–18.4) months. After SRT, 338 patients (73%) re-achieved a PSA <0.1 ng/ml, 126 (27%) retained higher values; 178 patients (38%) experienced progression and 30 men (6.5%) died ().
Figure 1. Kaplan–Meier curves of outcome after salvage radiotherapy for post-prostatectomy recurrent prostate cancer. Progression and overall survival in the entire cohort (N= 464), with 95% confidence intervals (A); stages pT2 versus pT3-4 (B); Gleason score GLS ≤6 versus GLS 7–10 (C); GLS ≤7 versus GLS 8–10 (D); metastatic disease (E); overall survival (F). In B–F, patients were stratified by the post-SRT PSA nadir.
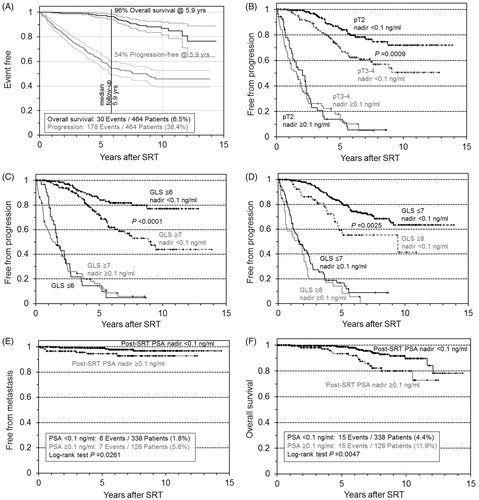
In univariate analysis, post-SRT progression correlated significantly with several risk factors (). Note the massive impact of achieving a post-SRT PSA <0.1 ng/ml. If the risk classifiers pT stage and Gleason score were stratified by the post-SRT PSA, then only patients who achieved an undetectable PSA retained a significant advantage with the better risk class (). In addition, R1 lost its advantage over R0 if patients retained a detectable PSA after SRT. Calculating from time of RP to endpoint/censoring confirmed the advantage from early SRT, that is, at PSA <0.2 ng/ml.
Table 2. Summary of univariate analyses of parameters that influence progression after salvage radiotherapy (SRT).
In multivariable Cox regression, the pre- and the post-RP PSA failed significance. shows a model (1) based on the remaining four pre-SRT parameters. If the post-SRT PSA response was incorporated in the analysis (model 2), then failing the nadir <0.1 ng/ml was the most important predictor of progression (HR = 8.3).
Table 3. Multivariable models for progression after salvage radiotherapy (SRT).
The pre-SRT PSA correlated with the post-SRT nadir: Failure to achieve an undetectable PSA occurred in 9% versus 36% of the patients with SRT at a PSA <0.2 ng/ml and ≥0.2 ng/ml, respectively (Chi-square test p < .0001). This is specifically important, as the post-SRT PSA was also significantly associated with metastatic disease () and overall mortality ().
We had no records of potential comorbidities of the patients. However, the median age at SRT of men who achieved a post-SRT undetectable PSA was 66.4 years versus 65.4 years in men who retained a PSA ≥0.1 ng/ml (t-test p > .7). Due to the limited number of events (N = 30), we did not conduct Cox regression analysis of OS.
Acute severe toxicity (grade 3 according to CTCAE version 3) was documented for two and three patients with gastrointestinal (GI) and genitourinary (GU) tract involvement, respectively. For both organ systems, grade-2 toxicity was recorded in 4.7% of the patients. Two men had late grade-3 reactions of the GI tracts, while severe GU tract toxicity was not observed. Late grade-2 complications occurred in 4.7% (GI tract) and 4.1% (GU tract), respectively, and 4.5% of the patients developed moderate (‘grade 2’) urethral stricture.
Discussion
The optimal timing of radiotherapy after RP is still under debate [Citation14,Citation15]. Three randomized clinical trials report a benefit from adjuvant radiotherapy (ART) compared with a wait-and-see strategy, and in one of them (SWOG 8794), not only biochemical progression but also overall survival was improved after ART [Citation2,Citation16,Citation17]. However, as salvage therapies in these trials are not well documented, even an indirect comparison of ART versus SRT is hampered. Randomized trials that compare the two strategies directly have not yet published results.
Clearly, ART but also SRT can be connected with overtreatment. In the observational arm of the ARO 96-02 trial, depending on risk factors, a quarter to half of the patients remained free from progression after 10 years. In a retrospective study on 450 men with post-RP biochemical progression but without adjuvant or salvage treatment before the development of metastases, the 5- and 10-year Kaplan–Meier estimates for metastasis-free survival were 67% and 48%, respectively, [Citation4]. The right setting for adjuvant versus early salvage radiotherapy is still under discussion, with respect to the patients’ clinical characteristics [Citation9]. Also the expected compliance to the strict follow-up required with wait-and-see must be taken into account.
SRT is estimated to require approximately 6 Gy more than ART [Citation18]. In the current analysis, we found SRT safe with median 66.6 (max. 72) Gy. After salvage IMRT, 76 Gy were connected with 3% late grade-3 side effects in a cohort of 89 patients [Citation19]. In a study on 285 patients, 165 of whom had IMRT with ≥70 Gy, the 5-year probability of grade-2 GI-tract toxicity was as low as about 2% with IMRT, while no grade-3 GI-tract events occurred in the entire cohort. GU-tract side effects did not seem to be reduced with IMRT, but despite the higher dose their incidence remained the same as with conventional 3D-conformal SRT [Citation20]. In the SAKK 09/10 trial with 344 patients, acute toxicity was compared after 64 versus 70 Gy. The higher dose was associated with a statistically insignificant rise in grade-2 and grade-3 GU tract, and grade-3 GI tract reactions, overall involving nine patients with severe events. In both trial arms, 44% of the patients received conventional 3D-CRT and 56% had IMRT/rotational techniques [Citation21]. Today, IMRT is the recommended standard for radiotherapy of PCa [Citation22].
In our cohort, including a minority of men with quite advanced pre-SRT PSA levels, a value ≥0.2 ng/ml significantly impaired the prognosis of patients with pT2, GLS≤ 6/GLS ≤7 or positive margins, that is, comparably favorable features according to univariate and multivariate analyses. International guidelines recommend SRT at a PSA of 0.5 ng/ml or less [Citation7]. The Mayo data are in full agreement with that cutoff [Citation10]. However, with advancing laboratory techniques, today a rising PSA is detectable at much earlier stages. Two multi-institutional studies that report on over 3100 patients, show a benefit from SRT at a PSA <0.2 ng/ml versus PSA 0.2–0.5 ng/ml versus higher values, one of them also in GLS subgroups [Citation15,Citation23]. Different from the above cohorts, we report exclusively on node-negative cases (pathologically confirmed in 97%) without ADT as part of the first salvage treatment.
A recent study on 716 node-negative patients with undetectable postoperative PSA examined the efficacy of SRT at PSA ≤0.5 ng/ml. Even in this limited range, the pre-SRT PSA was a major risk factor for biochemical recurrence in multivariable analysis (HR = 4.9 per unit PSA) besides pT3, GLS >8 and negative margins (HR values 2.1–2.7). However, the advantage from early SRT was biggest in patients with more unfavorable pathological features but less pronounced in men with lower-risk profiles [Citation24]. In an observational cohort of 259 patients, a benefit from additional ADT was found in men whose PSA exceeded 0.2 ng/ml [Citation25]. Regarding side effects, such early ADT is controversial.
Depending on the combination of risk factors, PSA values well below 0.1 ng/ml have already been suggested for the initiation of SRT [Citation8]. In fact, Freedland et al. reported that 93% of the patients (largely from the 1990s) whose post-RP PSA rose to 0.11–0.2 ng/ml had further progression within 3 years [Citation26]. Taken together, the above data underline the potential of very early SRT, that is, at a PSA <0.2 ng/ml, to improve the prognosis of post-RP recurrent PCa.
A relevance of the post-SRT PSA nadir for the patients’ outcome has been shown previously: Geinitz et al. reported on 96 men with a median pre-SRT PSA of 0.65 (range 0.2–4.0) ng/ml. With this unfavorable condition, only 66% achieved a post-SRT PSA <0.2 ng/ml. Despite the limited cohort size, multivariable analysis of cancer-specific and overall survival identified three significant risk factors: increasing GLS (2–6 versus 7 versus 8–10), post-SRT PSA >0.05 ng/ml and the requirement of neoadjuvant ADT, which was applied in 13% of the patients [Citation12]. In a study on 464 patients, Jackson et al. found an increased risk of biochemical progression, distant metastasis, cancer-specific and overall mortality in men who retained a PSA ≥0.1 ng/ml after SRT. Additionally, a detectable PSA nadir within 6 months post-SRT was an unfavorable prognostic parameter. Thus, high-risk patients could be characterized, for whom the authors suggest considering additional salvage therapy or at least an intensified follow-up [Citation13]. Regarding the potential side effects, the nadir could aid decision-making on ADT, which improved the outcome especially of patients with a higher pre-SRT PSA in the trials GETUG-16 and RTOG 96-01 [Citation27,Citation28]. With our increased database, we underpin our earlier findings of a predictive value of the nadir, specifically for overall survival [Citation11].
Shortcomings of our analysis relate to the retrospective design. We had to accept inhomogeneous follow-up sequences, both pre- and post-SRT that prevented a meaningful analysis of PSA kinetics, which is an informative and sensible risk marker [Citation5,Citation13]. PSA detection became more and more sensitive during the reported period. To prevent bias, we had to define a uniform range of undetectability, which does not reflect recent laboratory standards. Effects of the nadir below 0.1 ng/ml could thus not be determined. The dose prescription varied considerably, including dose-escalation which was applied during a couple of years in patients whose PSA declined during the course of SRT. This would introduce bias in an analysis of the dose dependence of our endpoints, which doubtlessly exists.
In our cohort of 464 post-RP recurrent PCa patients, we found that early SRT at a PSA <0.2 ng/ml is a safe and efficient means to re-achieve an undetectable PSA (<0.1 ng/ml), which improves freedom from progression, freedom from metastasis, and overall survival. Across pathological risk groups, a post-SRT undetectable PSA nadir characterizes men with lower rates of progression. As failing the nadir seems to predict an increased risk of metastasis and of overall mortality, affected patients may benefit from more frequent follow-up and/or from additional treatment like ADT.
Disclosure statement
The authors report no conflicts of interest.
Results in part was presented at the 57th annual ASTRO meeting, San Antonio 2015.
References
- Tollefson MK, Karnes RJ, Rangel LJ, et al. The impact of clinical stage on prostate cancer survival following radical prostatectomy. J Urol. 2013;189:1707–1712.
- Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66:243–250.
- Stephenson AJ, Kattan MW, Eastham JA, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostate-specific antigen era. JCO. 2009;27:4300–4305.
- Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109:32–39.
- Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. JCO. 2007;25:2035–2041.
- Wiegel T, Lohm G, Bottke D, et al. Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome–results of a retrospective study. Int J Radiat Oncol Biol Phys. 2009;73:1009–1016.
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–642.
- Mir MC, Li J, Klink JC, et al. Optimal definition of biochemical recurrence after radical prostatectomy depends on pathologic risk factors: identifying candidates for early salvage therapy. Eur Urol. 2014;66:204–210.
- Briganti A, Wiegel T, Joniau S, et al. Early salvage radiation therapy does not compromise cancer control in patients with pT3N0 prostate cancer after radical prostatectomy: results of a match-controlled multi-institutional analysis. Eur Urol. 2012;62:472–487.
- Stish BJ, Pisansky TM, Harmsen WS, et al. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol. 2016;34:3864--3871.
- Bartkowiak D, Bottke D, Thamm R, et al. The PSA-response to salvage radiotherapy after radical prostatectomy correlates with freedom from progression and overall survival. Radiother Oncol. 2016;118:131–135.
- Geinitz H, Riegel MG, Thamm R, et al. Outcome after conformal salvage radiotherapy in patients with rising prostate-specific antigen levels after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2012;82:1930–1937.
- Jackson WC, Johnson SB, Foster B, et al. Combining prostate-specific antigen nadir and time to nadir allows for early identification of patients at highest risk for development of metastasis and death following salvage radiation therapy. Pract Radiat Oncol. 2014;4:99–107.
- Bottke D, Bartkowiak D, Schrader M, et al. Radiotherapy after radical prostatectomy: immediate or early delayed? Strahlenther Onkol. 2012;188:1096–1101.
- Abugharib A, Jackson WC, Tumati V, et al. 'Very Early' salvage radiotherapy improves distant metastasis-free survival. J Urol. 2016;197:662–668.
- Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet. 2012;380:2018–2027.
- Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–962.
- King CR, Kapp DS. Radiotherapy after prostatectomy: is the evidence for dose escalation out there? Int J Radiat Oncol Biol Phys. 2008;71:346–350.
- Ost P, De Troyer B, Fonteyne V, et al. A matched control analysis of adjuvant and salvage high-dose postoperative intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1316–1322.
- Goenka A, Magsanoc JM, Pei X, et al. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur Urol. 2011;60:1142–1148.
- Ghadjar P, Hayoz S, Bernhard J, et al. Acute toxicity and quality of life after dose-intensified salvage radiation therapy for biochemically recurrent prostate cancer after prostatectomy: first results of the randomized trial SAKK 09/10. JCO. 2015;33:4158–4166.
- Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative Intent. Eur Urol. 2017;71:618–629.
- Tendulkar RD, Agrawal S, Gao T, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34:3648--3654.
- Fossati N, Karnes RJ, Cozzarini C, et al. Assessing the optimal timing for early salvage radiation therapy in patients with prostate-specific antigen rise after radical prostatectomy. Eur Urol. 2016;69:728–733.
- Ervandian M, Hoyer M, Petersen SE, et al. Salvage radiation therapy following radical prostatectomy. A national Danish study. Acta Oncol. 2016;55:598–603.
- Freedland SJ, Sutter ME, Dorey F, et al. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology. 2003;61:365–369.
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17:747–756.
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med. 2017;376:417–428.
