Abstract
Objective: To identify a failure site-specific prognostic model by combining immunohistochemistry (IHC) and molecular imaging information to predict long-term failure type in squamous cell carcinoma of the head and neck.
Patient and methods: Tissue microarray blocks of 196 head and neck squamous cell carcinoma cases were stained for a panel of biomarkers using IHC. Gross tumor volume (GTV) from the PET/CT radiation treatment planning CT scan, maximal Standard Uptake Value (SUVmax) of fludeoxyglucose (FDG) and clinical information were included in the model building using Cox proportional hazards models, stratified for p16 status in oropharyngeal carcinomas. Separate models were built for time to locoregional failure and time to distant metastasis.
Results: Higher than median p53 expression on IHC tended toward a risk factor for locoregional failure but was protective for distant metastasis, χ2 for difference p = .003. The final model for locoregional failure included p53 (HR: 1.9; p: .055), concomitant cisplatin (HR: 0.41; p: .008), β-tubulin-1 (HR: 1.8; p: .08), β-tubulin-2 (HR: 0.49; p: .057) and SUVmax (HR: 2.1; p: .046). The final model for distant metastasis included p53 (HR: 0.23; p: .025), Bcl-2 (HR: 2.6; p: .08), SUVmax (HR: 3.5; p: .095) and GTV (HR: 1.7; p: .063).
Conclusions: The models successfully distinguished between risk of locoregional failure and risk of distant metastasis, which is important information for clinical decision-making. High p53 expression has opposite prognostic effects for the two endpoints; increasing risk of locoregional failure, but decreasing the risk of metastatic failure, but external validation of this finding is needed.
Introduction
The emergence of human papilloma virus (HPV) related oropharyngeal carcinomas as a new, prognostically favorable [Citation1–4] entity among head and neck squamous cell carcinoma (HNSCC) has led to a flurry of treatment de-escalation trials of HPV + patients [Citation5]. As a result, p16 as a surrogate for HPV infection may become the first example of a predictive biomarker guiding management decisions in HNSCC. It has also been shown that genetic and immunohistochemical (IHC) biomarker profiles differ according to HPV status [Citation6,Citation7]. However, despite intensive research, there is a paucity of useful biomarkers in the non-HPV-related cases, etiologically linked with a history of heavy tobacco and alcohol use [Citation8].
Prognostic models in the literature often focus on composite endpoints, such as disease-free survival or time to any failure [Citation9–12]. There is a twofold downside of using such composite endpoints; first, combining distant and locoregional failure (LRF) in one single analysis may result in the loss of information about the biology of the disease. Second, it is of substantial interest to know if the patient's risk is dominated by distant metastasis (DM) or LRF in the clinical management of HNSCC and the pooling of endpoints in composite analysis prohibits the extraction of such information. We have previously hypothesized [Citation13] that some biomarkers may be associated with a specific type of failure in HNSCC. This is potentially of interest because it could lead to individualization of treatment based on each patient’s failure risk.
In the current analysis, we focus on combining IHC assays and molecular imaging assays to build separate prognostications for DM and LRF. As a preplanned substudy, we intended to validate a previously published prognostic model on survival [Citation14] on which the selection of IHC biomarkers were based rather than on a biologic rationale.
Material and methods
This is a retrospective cohort study (n = 287) of consecutive Danish adults diagnosed and receiving (chemo-) radiotherapy for previously untreated, locally advanced HNSCC in a single center according to national guidelines made by the Danish Head and Neck Group (http://www.dahanca.dk). The population has previously been described in detail [Citation15–17].
The inclusion criteria were as follows: (1) diagnosis and intensity-modulated radiotherapy treatment for HNSCC between January 2005 and October 2009; (2) sufficient amount of diagnostic biopsy material to include in a tissue microarray (TMA); (3) a pretreatment 18F-FDG PET/CT scan performed as part of radiotherapy planning.
The study was approved by both the Capital Regional Committee on Health Research Ethics (ID: H-2-2013-122) and the Data Protection Authority (Journal no. 30-1129). The Danish registry for ‘Tissue Usage’ had no records of any of the included patients opposing to their tissue being used for research purposes.
Tissue microarray and immunohistochemistry
Pre-treatment diagnostic hematoxylin–eosin (H&E) stained sections were reevaluated for relevant and representative tumor tissue by one of the authors (GBR) and validated by an experienced pathologist with head and neck cancer sub specialization (MHT). TMA blocks were constructed from 2 mm core punch outs from each patient’s pretreatment, formalin-fixed paraffin-embedded (FFPE) diagnostic tumor biopsy blocks, stored since diagnosis at room temperature in the archive of the Department of Pathology. Due to sparseness of diagnostic tissue in many of the diagnostic biopsies, multiple coring was not possible. The part of the cohort (n = 98) collected after a previously published pilot series (n = 102) [Citation15] was stained at the Department of Pathology, Rigshospitalet, Copenhagen, Denmark, except for glutathione-S-transferase-π (GST-π) and β-tubulin-1 and β-tubulin-2 which were stained manually at the Greenebaum Comprehensive Cancer Center, Baltimore, MD.
Sections of 4–5 µm were cut from the TMA blocks and mounted on positively charged glass slides, air-dried overnight and incubated at 60 °C for 60 min (Thermo Scientific section dryer, Waltham, MA, USA). Fully automated IHC stains were performed according to standard guidelines of the pathology departments and according to the protocols of the manufacturers of the primary antibodies including staining of relevant control tissues (tonsil, colon, pancreas and liver, lung) in the same batch. For the 102 patients described in the pilot series [Citation15], fully automated IHC stains were performed on ‘Leica Bond III’ instruments staining for Bcl-2 (clone bcl-2/100/D5, Leica Biosystems, Newcastle, UK), p53 (clone DO-7, Leica Biosystems), and p16 (clone E6H4, Ventana Medical Systems, Inc. Tucson, AZ). Staining for EGFR, (ready to use Clone 5B7, Ventana Medical Systems) and Ki-67 (clone MIB1, DAKO, Glostrup, Denmark) were performed on the fully automated BenchMark ULTRA IHC/ISH Staining Module (Ventana Medical Systems).
The remaining 98 patients were stained using BenchMark ULTRA IHC/ISH Staining Module, using sections of the TMAs cut at four micron thickness, mounted on Dako FLEX IHC microscope slides. Staining was performed using the following clones: Bcl-2 (clone 124, DAKO), p53 (clone DO-7, DAKO), p16 (clone E6H4, Ventana Medical Systems), EGFR (ready-to-use Clone 5B7, Ventana Medical Systems), Ki-67 (clone MIB1, DAKO). Relevant control tissues, tonsil, colon, pancreas, liver and lung for the staining of p53, Ki-67, Bcl-2, p16, were costained in each batch. Both the GST-π and the β-tubulin stains had internal controls in the HNSCC tissue. Negative controls were performed by omitting the primary antibody.
Manual IHC was performed for the GST-π (clone LW29, Leica Biosystems, Newcastle, UK) and β-tubulin-1 and β-tubulin-2 (clone JDR.3B8, Santa Cruz Biotechnology, Inc., Dallas, TX) stains as previously described [Citation15].
The first of the consecutive TMA sections from each TMA block was stained with H&E to ensure the presence of relevant and sufficient amount of tumor tissue in each core.
The quantification of the immunostained TMA sections were performed following D.C. Allred [Citation18]. The Allred scoring consists of a proportion score (0–5; 0: 0% positive tumor cells; 1: up to 1% positive tumor cells; 2: up to 10% positive tumor cells; 3: up to 33% positive tumor cells, 4: up to 66% positive tumor cells; 5: up to 100% positive tumor cells) which is added to an intensity score (0–3; 0: no staining of tumor cells; 1: weak; 2: moderate; 3: intense) for the total score of (0; 2–8). IHC stains were scored by a single observer (GBR), supervised by another author (MHT). Difficult cases were discussed and consensus was reached using a double-headed microscope. The clinical patient data and the treatment outcome were blinded to GBR and MHT until the IHC scoring had been completed.
For statistical analysis, IHC variables were dichotomized according to the population median of the total Allred score (except for p16, which was scored according to EORTC/DAHANCA guidelines [Citation19], regardless of tumor location). In short, to be classified as p16 positive the staining must be strong and uniform in more than 70% of carcinoma cells in both nucleus and cytoplasm [Citation19].
During the IHC-staining process, some patients’ TMA cores were lost, fragmented, used up cutting through the block or otherwise unable to being scored. If more than two of a patient’s IHC stains were non-evaluable, the patient was excluded from the current analysis.
FDG PET/CT imaging and delineation
As part of the radiotherapy planning, integrated 18F-FDG PET/CT scans were performed on fasting patients as described recently [Citation17]. Immobilized patients in individually molded masks were scanned one hour after the injection of the 18F-FDG tracer in accordance with the department’s guidelines. Intravenous contrast was given unless contraindicated. The standard uptake values (SUV) were calculated to correct for bodyweight and injected activity. PET/CT scans were used for delineation. A multibed PET scan was performed after the CT, covering the area from vertex to mid-thigh.
Delineation of the 18F-FDG PET positive area was performed by a nuclear medicine physician; the gross tumor volume (GTV) from the CT scan was delineated by a radiologist in collaboration with an oncologist.
Statistics
An attempt to perform an external validation of a previously published IHC-based model [Citation14] was made. The methods used in the original publication were applied to the present dataset. The endpoint in this analysis was overall survival. Quantification of IHC was performed as stated in the original paper.
Multivariable failure-type specific Cox models were built using following candidate predictors and coding: Concomitant cisplatin prescribed (no = 0; yes = 1), tobacco use (never smoker: 1; former smoker: 2; current smoker: 3), the natural logarithm of the GTV measured continuously in cubic centimeters (GTV (ln)) and the maximal SUV (SUVmax (ln)) (continuous, natural logarithm of SUVmax)). IHC candidate predictors were dichotomized stains of Bcl-2, p53, GST-π; cytoplasmic β-tubulin-1; nuclear β-tubulin-2, EGFr, Ki-67 as described above. Patients were stratified between p16-positive oropharyngeal squamous cell carcinoma (p16 + OPSCC) versus others. The ‘other’ stratum includes the patients with p16-negative OPSCC and all patients with SCC in subsites other than oropharynx. p16 status was used as stratification leading to p16 status-specific baseline hazards, but no HR for p16 positivity versus p16 negativity (proportionality not assumed).
Multivariable model reduction was performed by backwards elimination using the Wald score statistics, retaining parameters with a significance level of p < .15. Two-year patient-specific Kaplan–Meier (KM) estimates of time to locoregional failure (TLRF)/time to distant metastasis (TDM) were calculated based on the reduced multivariate models. A predefined analysis plan described codings, backwards elimination procedure and stratification.
LRF was defined as failure in either the original tumor site or in the regional lymph nodes with censoring for DM or death. LRF or death was treated as censorings in analysis of TDM. Patients with synchronous LRF and DM failure were included as events in both models in accordance with a predefined analysis plan. Two-year KM estimates for both TLRF and TDM of the reduced multivariate models were calculated.
SPSS version 22 (IBM Armonk, NY, USA) was used for statistical analysis, supplemented with R version 3.3.0 and R-studio version 0.99.896 (Boston, MA, USA).
Results
The characteristics of the included patients are shown in . A total of 287 patients were eligible for the collection of diagnostic biopsies. Eighty-four patients were excluded due to scarcity of tumor material in the primary diagnostic biopsies. A total of 203 patients' samples were included in the TMA analysis. Another seven patients (4 from the pilot series, 3 from the present series) were excluded from analyses after IHC staining because of missing stains due to scarcity of actual tumor tissue in the TMA sections, or due to cores falling off the slide during the staining process. A total of 196 patients were included in the final model building analyses (). Intensity of some of the included IHC-stains (p53 and Bcl-2) was missing in one patient, and this patient was excluded from the validation attempt and the p53-based patient characteristics. Median follow-up in censored patients was 32 months.
Figure 1. Patient flowchart: IMRT: intensity-modulated radiotherapy; FFPE: formalin-fixed paraffin-embedded; TMA: tissue microarray; IHC: immunohistochemistry.
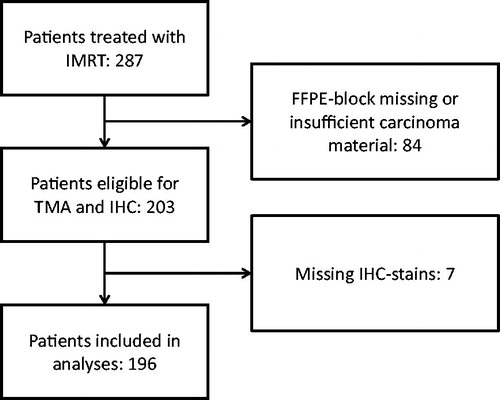
Table 1. Patient characteristics based on p53 expression.
The attempted validation of the IHC-based model performed by Cullen et al [Citation14] is shown in . The prognostication from the Cullen model could not be reproduced.
Figure 2. Kaplan–Meier curves of overall survival by number of positive IHC biomarkers. Sum of positive IHC-stains as performed in the reference [Citation14].
![Figure 2. Kaplan–Meier curves of overall survival by number of positive IHC biomarkers. Sum of positive IHC-stains as performed in the reference [Citation14].](/cms/asset/b3255655-6833-49e0-ae94-358973fcda87/ionc_a_1364870_f0002_c.jpg)
Failure site-specific cox models
The failure site-specific Cox models are shown in . The models for the two failure types are different, suggesting a possibility to distinguish between locoregional and distant failure. Most importantly, high expression of p53 is found of borderline significance for increased risk of LRF (HR = 1.9, 95% CI [0.99; 3.77]) but significant for decreased risk of DM (HR = 0.23, 95% CI [0.065; 0.83]) with a χ2 p value of p = .003 for differential effect, cf. . This p value remains significant after Bonferroni correction for the 11 comparisons. Cumulative incidence curves (1-KM) for high/low p53 expression in the two strata are shown in .
Figure 3. Forest plot of hazard ratios for locoregional failure (closed circles, full line, top) versus distant metastatic failure (open circles, broken line, bottom) of each of the investigated covariables in the current study. Data are from the reduced models presented in when included, or from the full model if not included in the reduced model (marked with dagger and gray color on the forest plot). χ2 p values for differential effect on the two endpoints are given, the p value of p .003 for p53 is significant after correcting for multiple comparisons using the Bonferroni method.
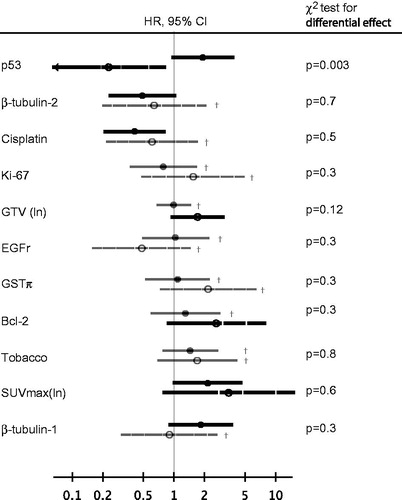
Figure 4. Cumulative incidence curves (1-KM) for locoregional failure and distant metastasis in the two strata (p16-positive oropharyngeal cancers and ‘others’). The ‘other’ stratum includes the patients with p16-negative OPSCC and all patients with SCC in subsites other than oropharynx.
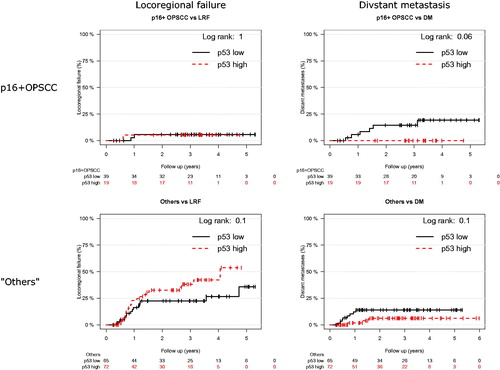
Table 2. Failure site-specific Cox models.
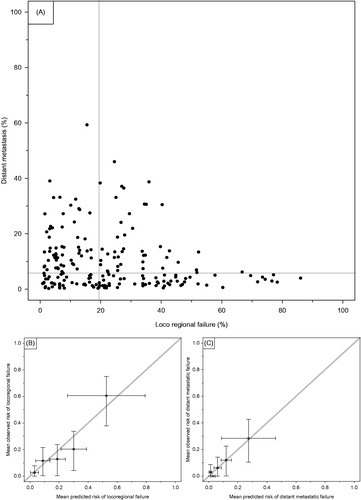
A visual presentation of individual risk estimates based on the two-year KM estimates of the site-specific reduced multivariate models is shown in . The suggested possibility to distinguish LRF and DM is reflected in the dispersion in distribution. show predicted versus observed cumulative incidence of the two outcomes.
Figure 5. (A) Cumulative incidence (1-KM) plot of estimates for 2-year risk of DM or LRF from multivariate reduced models. Each patient had risk estimates of the two failure types plotted against each other. The gray lines represent the medians of the respective 1-KM estimates. (B) and (C) calibration plots of observed versus predicted cumulative incidence (1 minus two-year KM estimate) of risk of the two failure types. The patients were grouped in quintiles based on their predicted two-year estimates from the reduced multivariate models.
Discussion
The main purpose of this study was to combine imaging and IHC biomarkers in an attempt to distinguish between risk of LRF and risk of DM. A failure-specific model would be more clinically useful than prognostication in itself as it would allow selection of patients for the most promising experimental treatment strategies – that is, intensified local therapy for patients with high risk of LRF and intensified systemic therapy for patients with a high risk of DM. Also, we hypothesized that failure-type specific models may add biological insights compared to composite endpoints.
Our main worry at the onset of the project was the risk of not being able to distinguish between the two types of failures, if patients with a high risk of LRF would generally also be at high risk of DM. shows that our model does indeed identify patients at a high risk of LRF and low risk of DM and vice versa as a result of the selective risk profiling of p53 and other markers, cf. and . This is indeed promising for future research moving away from pure prognostic models and into models capable of distinguishing between the risk of different failure types for individual patients, thus allowing a tailored package of locoregional and systemic therapy in the individual patient. However, the limited sample size – in particular the low number of distant failure events – and the lack of external validation should be acknowledged as limitations.
The main driver of the failure site prediction was p53 status, indicating a higher risk of LRF and lower risk of DM in the current dataset. While it should be acknowledged that the dataset is limited and external validation would be required to draw firm conclusions, the result is intriguing. When comparing with the literature, studies distinguishing between LRF and DM are rare [Citation13] and none of the previously investigated biomarkers had an as distinct different prognostic value on locoregional versus distant failures as p53 in this study, albeit direct comparison is difficult as model building, staining procedures and quantifications of IHC stains are different. Quite a few studies have focused on p53 and with some discrepancy in conclusions; immunohistochemical expression of p53 has previously been shown to predict locoregional failure in the head and neck area [Citation20,Citation21]. Koch et al. [Citation20] found an increased risk of locoregional failure, but no effect of TP53 mutation on the overall survival. Ma et al. [Citation21] found that mutated p53 gene, but not overexpression of the protein, could be a useful prediction tool for local tumor recurrences. In other words, the literature suggests that p53 overexpression may be correlated to higher risk of LRF, but to our knowledge, this study is the first to associate high expression of p53 with reduced risk of DM, as found in the present material.
Traditionally, p53 expression has been associated with SCC typically located in the larynx or hypopharynx and characterized by heavy use of tobacco and alcohol [Citation22,Citation23] and with no HPV infection. This is in accordance with the demographics of where it is seen that the p53 high expression and p53 low expression patient groups differ, most importantly with fewer patients with high p53 expression in p16 + OPSCC-group. Note, however, that the analyses in the present study are stratified by p16 status and so the effect of p16 + in OPSCC is already accounted for in the analyses.
In the analyses, tobacco use was found to increase the risk of both outcomes, but not significantly so. Other studies have found smoking status significantly prognostic [Citation1]. Most likely, the lack of significant association in the study is due to the limited power – it should be noted that the point estimate of the full model in is in good agreement with the literature data.
The data also allowed for a preplanned external validation of a previously published prognostic model [Citation14]. Such external validations are much needed and called for by several important papers [Citation24,Citation25]. Unfortunately, the previously published model [Citation14] did not provide good prognostication of overall survival in our series. The differences in treatment between the studies should definitely be acknowledged; most importantly the patients in the present study did not receive induction chemotherapy. This could be one explanation of the discrepancy. Another difference that might explain some of the discrepancy is the use of a different IHC clone used to stain for GST-π. In the present analysis, clone LW29 (Leica Biosystems) was used, whereas in the original model clone, 353-10 was used (Dako, Carpinetria, CA, USA). Due to the original manufacturers cessation of production of the original clone, this could not be obtained. Also, in our cohort, 22 nasopharyngeal patients were included – a site not included in the Cullen paper. Nevertheless, the result is disappointing, as a strong prognostic model should be robust across different patient series in order to be clinically useful. The Cullen prognostic model has not been externally validated. Well described challenges with this kind of modeling are overfitting of included variables.
The limitations of the present study should be recognized. The sample size is limited and in particular the number of metastatic failures is modest. Nevertheless the present study demonstrates a method to distinguish between competing types of failure of utmost clinical relevance and the data suggest that such separation between risks is indeed possible with commonly available IHC techniques. Furthermore, the separate analysis of risk of locoregional failure and distant metastasis revealed intriguing biological information and warrants a deeper analysis, using for example DNA sequencing to elucidate the underlying reasons for the different recurrence pattern according to p53 status.
Acknowledgments
The authors group are grateful to histotechnician Pernille Frederiksen, Rigshospitalet, histotechnicians accredited by the American Society of Clinical Pathology (ASCP) Kimberly Tuttle and Loretta Kendall, UMSOM, for helping with the IHC staining. The departments of pathology in the Capital Region of Denmark (Rigshospitalet, Herlev, Hvidovre), and in Region Zealand (Roskilde, Slagelse, Naestved) are acknowledged for helping with retrieval of the diagnostic FFPE material.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
- Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269.
- Lassen P, Eriksen JG, Hamilton-Dutoit S, et al. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998.
- Lewis JS, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–1096.
- Mirghani H, Amen F, Blanchard P, et al. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: ongoing trials, critical issues and perspectives. Int J Cancer. 2015;136:1494–1503.
- Seiwert TY, Zuo Z, Keck MK, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21:632–641.
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582.
- Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22.
- Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:3930–3938.
- Kikuchi M, Koyasu S, Shinohara S, et al. Prognostic value of pretreatment 18 F-fluorodeoxyglucose positron emission tomography/CT volume-based parameters in patients with oropharyngeal squamous cell carcinoma with known p16 and p53 status. Head Neck. 2015;37:1524–1531.
- Shiga H, Heath EI, Rasmussen AA, et al. Prognostic value of p53, glutathione S-transferase pi, and thymidylate synthase for neoadjuvant cisplatin-based chemotherapy in head and neck cancer. Clin Cancer Res. 1999;5:4097–4104.
- Shinohara S, Kikuchi M, Tona R, et al. Prognostic impact of p16 and p53 expression in oropharyngeal squamous cell carcinomas. Jpn J Clin Oncol. 2014;44:232–240.
- Ataman OU, Bentzen SM, Wilson GD, et al. Molecular biomarkers and site of first recurrence after radiotherapy for head and neck cancer. Eur J Cancer. 2004;40:2734–2741.
- Cullen KJ, Schumaker L, Nikitakis N, et al. beta-Tubulin-II expression strongly predicts outcome in patients receiving induction chemotherapy for locally advanced squamous carcinoma of the head and neck: a companion analysis of the TAX 324 trial. J Clin Oncol. 2009;27:6222–6228.
- Rasmussen GB, Vogelius IR, Rasmussen JH, et al. Immunohistochemical biomarkers and FDG uptake on PET/CT in head and neck squamous cell carcinoma. Acta Oncol. 2015;54:1408–1415.
- Due AK, Korreman S, Bentzen SM, et al. Methodologies for localizing loco-regional hypopharyngeal carcinoma recurrences in relation to FDG-PET positive and clinical radiation therapy target volumes. Acta Oncol. 2010;49:984–990.
- Rasmussen JH, Vogelius IR, Fischer BM, et al. Prognostic value of 18F-fludeoxyglucose uptake in 287 patients with head and neck squamous cell carcinoma. Head Neck. 2015;37:1274–1281.
- Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168.
- Lassen P. DAHANCA/EORTC guidelines for scoring and classification of p16-immunohistochemistry in HPV-related oropharyngeal cancer [Internet]. 2012 [cited 2015 May 10]. Available at: http://www.dahanca.dk/get_media_file.php?mediaid=322.
- Koch WM, Brennan JA, Zahurak M, et al. p53 mutation and locoregional treatment failure in head and neck squamous cell carcinoma. J Natl Cancer Inst. 1996;88:1580–1586.
- Ma L, Ronai A, Riede UN, et al. Clinical implication of screening p53 gene mutations in head and neck squamous cell carcinomas. J Cancer Res Clin Oncol. 1998;124:389–396.
- Brennan JA, Boyle JO, Koch WM, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:712–717.
- Zhou G, Liu Z, Myers JN. TP53 mutations in head and neck squamous cell carcinoma and their impact on disease progression and treatment response. J Cell Biochem. 2016;11:1–11.
- McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–9072.
- Moons KGM, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–W73.
