 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background and purpose: The galactose analog 2-[18F]fluoro-2-deoxy-d-galactose (FDGal) is used for quantification of regional hepatic metabolic capacity by functional positron emission tomography computerized tomography (PET/CT). In the present study, FDGal PET/CT was used for functional treatment planning (FTP) of stereotactic body radiotherapy (SBRT) of liver metastases with the aim of minimizing radiation dose to the best functioning liver tissue.
Material and methods: Fourteen patients referred for SBRT had FDGal PET/CT performed before and one month after the treatment. The planning CT and the FDGal PET/CT images were deformable co-registered.
Results: A reduction in the mean dose of approximately 2 Gy to the best functioning sub-volumes was obtained. One patient developed grade 2 acute morbidity and no patients experienced grade 3 or higher acute morbidities. The regional hepatic metabolic function post-treatment was linearly correlated to the regional radiation dose and for each 10-Gy increase in dose (γ10Gy), the metabolic function was reduced by 12%. A 50% reduction was seen at 22.9 Gy in 3 fractions (CI 95%: 16.7–30.4 Gy).
Conclusion: The clinical study demonstrates the feasibility for FTP in patients with liver metastases and it was possible to minimize the radiation dose to the best functioning liver tissue.
Introduction
Primary and secondary liver cancer is among the most frequent causes of cancer death in the world [Citation1,Citation2]. The tumors are treated with increasing aggressiveness with surgery as the preferred treatment option [Citation3,Citation4], but a proportion of the patients are not eligible for resection for technical or medical reasons. In such cases, stereotactic body radiotherapy (SBRT) is often an appropriate therapeutic option [Citation5,Citation6]. Cohort studies with long follow-up have demonstrated that SBRT results in local tumor control rates of 70–90% and that long-term survival can be achieved [Citation7,Citation8]. However, the risk of radiation induced liver disease (RILD), a feared complex condition with a broad range of clinical symptoms ranging from asymptomatic elevation of liver enzymes to liver failure and death, is the reason for the cautious approach for SBRT of liver cancer [Citation9]. Presence of parenchymal liver disease such as hepatitis B infection and cirrhosis increases the risk of RILD [Citation9].
In this clinical study, functional treatment planning (FTP) was used to minimize the radiation dose to the best functioning sub-volumes of liver tissue [Citation10,Citation11]. Regional hepatic metabolic function, in terms of galactokinase capacity, was quantified using positron emission tomography (PET) [Citation12,Citation13] with the hepatocyte-specific tracer, 2-[18F]fluoro-2-deoxy-d-galactose (FDGal) [Citation14].
The aim was to test the feasibility and safety of FTP-SBRT in patients with colorectal cancer (CRC) liver metastases by minimizing the radiation dose to sub-volumes of best functioning liver tissue. Furthermore, we aimed at developing a radiation dose-response relationship for hepatic metabolic function.
Material and methods
Patients
Fifteen patients were consecutively included in the study; one patient withdrew his consent. All the patients had CRC liver metastases and none had any laboratory or clinical manifestations of parenchymal liver disease. Prior to inclusion, a multidisciplinary liver tumor board had evaluated the patients and deemed them ineligible for surgery or radiofrequency ablation (RFA) for medical or technical reasons. The study was approved by the Central Denmark Region Committees on Health Research Ethics and conducted in accordance with the Helsinki Declaration II. Written informed consent was obtained from all patients. The study was registered at clinicaltrials.gov (NCT01861873).
FDGal PET/CT
FDGal was produced at the PET Centre’s own radiochemistry laboratory. The patient was positioned in supine position in a custom-made Vac-loc (SBF II, VacFix, Par Scientific, Denmark) on the flat scanner bed of a Siemens Biograph TruePoint PET/CT scanner (Siemens AG, Erlangen, Germany). A topogram of the upper abdomen was acquired for optimal positioning of the liver within the trans-axial field of view of the PET camera (21.6 cm). For definition of anatomical structures and attenuation correction of PET data, a low-dose CT scan was performed with 3 mm slice thickness. 100 MBq (range: 96–123 MBq) FDGal was injected intravenous as a bolus in 10 mL saline over 20 s in the beginning of a 20 min dynamic PET scan. PET data were reconstructed using an iterative method with resolution modeling (336 matrix, voxel 2 × 2 × 2 mm2) and a time-frame structure of 20 × 5, 1 × 10, 3 × 20, 1 × 30, 1 × 40, 2 × 60 and 7 × 120 seconds (total 20 min) was used. Standard uptake value (SUV) of FDGal was calculated by normalizing the tissue concentration for body weight and injected dose. Images of the averaged SUV for the last 10 min of the dynamic scan were constructed and used as basis for the further analyzes. The baseline FDGal PET/CT was used for FTP and the FDGal PET/CT performed one month after SBRT was used for assessment of the radiation dose-response relationship.
SBRT
The SBRT technique is described in details in previous publications [Citation8]. Patients were immobilized in supine position in the above-mentioned Vac-loc whole-body fixation. Eight patients had gold fiducial markers implanted near the tumor and were treated with abdominal compression to reduce the respiratory tumor motion and six patients had Calypso transponders (Calypso Soft Tissue Beacon Transponders, Varian Medical Systems, Palo Alto, CA, USA) implanted and were treated with Calypso-guided gating without abdominal compression. As standard, a four-dimensional (4D)-CT scan and a contrast enhanced (Visipaque 270; 2 mL/kg) breath-hold CT scan was acquired. The clinical target volume (CTV) was identical to the gross tumor volume (GTV) and defined on the contrast enhanced CT scan. A 5 mm margin in the transversal plan and a 7–10 mm margin in the cranio-caudal direction were added to the CTV to create the planning target volume (PTV). For the patients included in the Calypso study, the treatment was planned on breath-hold and contrast enhanced CT scan and the patients were treated with free-breathed gating in the expiratory phase. A detailed description of the Calypso technique is given elsewhere [Citation15]. The CT scan was reconstructed with 3 mm slice thickness. Treatment planning was performed using Eclipse treatment planning system (Varian Medical Systems Version 11.0, Palo Alto, CA, USA).
The baseline FDGal PET/CT images of the liver were divided into nine sub-volumes based on the absolute voxel values of SUV for FDGal. The 10% (± 10%) (V’∼10%) of the liver with the highest SUV was defined as the ‘best’ functioning liver tissue, the volume denoted V’∼20% was the volume with the 20% (±10%) highest SUV, V’∼30% with the 30% highest SUV etc. The mean dose (Dmean) delivered to V’∼10%, V’∼20% and V’∼30% were denoted D’∼10%, D’∼20% and D’∼30%, respectively. For practical reasons, the sub-volumes used for the optimization process were V’∼10% V’∼20% and V’∼30%. The CTV was subtracted from all volumes (VLiver, V’∼10% V’∼20% and V’∼30%) and the structures were transferred to the planning CT using deformable co-registration (Smartadapt, Varian Medical Systems Version 11.0, Palo Alto, CA, USA/MIM Software Version 6.5 (MIM Software Inc, Cleveland, OH, USA)). The implanted fiducial markers were used for quality assurance of the deformable co-registration of the FDGal PET/CT and planning CT images. The marker positions from the two scans matched within 5 mm.
The prescribed Dmean to the CTV was 45–60 Gy in 3–6 fractions with CTV enclosed by 95% and the PTV by the 67% isodose. The constraints for organs at risk were the following: 700 cc liver tissue should receive less than 15 Gy, D1cc of stomach, esophagus, duodenum and colon should be kept below 28 Gy, Dmax of spinal cord <18 Gy, D35% of both kidneys <15 Gy and 50% of the ipsilateral kidney <15 Gy. As objective, it was aimed to keep the Dmean of the liver under 15 Gy and the D1cc of the heart under 30 Gy. The SBRT was performed with a 7-field intensity-modulated radiotherapy (IMRT) technique including non-coplanar beam angels. In selection of beam-angles, minimum overlap of entrance paths with V’∼10%, V’∼20% and V’∼30% was prioritized, while maintaining appropriate beam-angles with respect to target and organs at risk. During the IMRT optimization process, upper constraints were set on V’∼10%, V’∼20% and V’∼30%. The constraints were individualized to each patient and adjusted so that the target coverage and maximum dose to critical normal tissue was not compromised.
The FTP-SBRT was delivered with a TrueBeam linear accelerator (Varian Inc, Palo Alto, CA, USA). A cone-beam CT (CB-CT) scan was acquired prior to each fraction for accurate set-up of the patient. Implanted fiducial markers were used for co-registration between the CB-CT and the planning CT scan.
Morbidity
The morbidity was registered at baseline, one and three month after treatment by use of the CTCAE 4.0 grading scale. Any increase in grade from baseline was considered possibly related to the FTP-SBRT.
Statistics
For investigation of the radiation dose-response relationship the FDGal PET/CT performed one month after SBRT was used and data were analyzed by deviding the liver in 5-Gy intervals. To perform the further analyzes as continuous data, the mean dose in each radiation dose interval was used (2.5 Gy, 7.5 Gy etc). Data were normally distributed based on histograms and quantile plots.
Individual mean value of SUV (SUVmean) in each radiation dose interval was normalized to percentage to perform a population-based analysis using the equation below [Citation16]:
SUVmin and SUVmax represent the minimum and maximum SUV in the liver for the individual patient. The assumption for this normalization was that the metabolic function of the liver tissue that received a dose close to zero was functioning 100%. Furthermore, the metabolic function of the liver that received a dose corresponding to the prescribed CTV dose was functioning 0%. This was supported by almost identical SUVmean in well-controlled RFA necrosis as SUVmean in the CTV.
A linear regression model (mixed model [Citation17]) was applied to reveal a dose-response relationship and a sigmoid-shaped fit of data was also tested. Three patients were treated with six fractions, and the biologically effective dose (BED) was calculated for the three patients with α/β = 3. The radiation dose was recalculated in three fractions and V<15Gy is corrected for the three patients.
The difference between non-irradiated sub-volumes of the liver (< 3 Gy) between the two FDGal PET scans was compared for each patient.
All analyses were performed using STATA version 13 software.
Results
Ten patients were treated with FTP-SBRT for a solitary CRC metastasis and four patients for two metastases. Eight patients (57%) had previously undergone surgical resection (SR) (n = 5), RFA (n = 5) or SBRT (n = 5); six patients had received a combination of more than one local treatment ().
Table 1. Patient characteristics.
There was only observed mild morbidities related to the FTP-SBRT; no severe (grade 3-5) acute morbidity was registered. Fifteen grade 1 morbidities and one grade 2 fatigue were experienced. This patient experienced fatigue after the first administration of adjuvant chemotherapy (Supplementary Table 1).
At the last follow-up (mean 16.6 months), 10 patients were alive and four patients died due to progression of the cancer. Six patients were without local recurrence or distant metastases in the liver. Local recurrence was observed in one of the 18 treated tumors.
All FTP-SBRT plans met the pre-defined dose-volume constraints with the exception of the soft constraint to keep Dmean(Liver) below 15 Gy, which was not met in four patients (). shows the dose-volume histograms (DVH) and the CT scan with the sub-volumes of best functioning liver tissue (V’∼10%, V’∼20% and V’∼30%) receiving lower radiation doses than the VLiver in patient #10.
Figure 1. Dose-volume histogram (DVH) of VLiver, V’∼10%, V’∼20% and V’∼30% in patient #10 treated with FDGal PET/CT based FTP-SBRT with a total dose of 56.25 Gy in three fractions (A). The spatial distribution of FDGal (V’∼10%, V’∼20% and V’∼30%) in the liver displayed together with the dose color wash of 15 Gy (B).
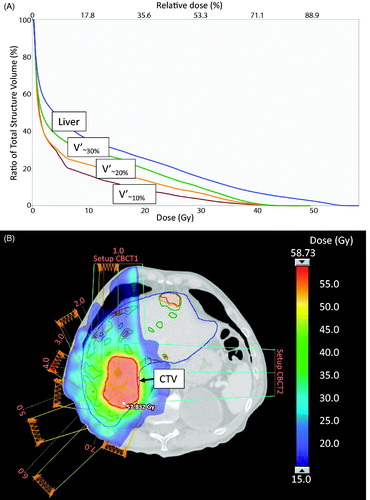
Table 2. Individual dose volume parameters.
The optimization sub-volumes V’∼10%, V’∼20% and V’∼30%, which were defined by the distribution of the SUV in the liver, varied considerably and did not relate to the VLiver, CTV, PTV or any of the dose-volume parameters ().
For all patients, FTP-SBRT resulted in lower Dmean to the sub-volumes of best functioning liver tissue with mean values of D’∼10%, D’∼20% and D’∼30% of 8.6, 8.5 and 8.2 Gy, respectively, which was low compared to the Dmean(Liver) of 10.4 Gy. In individual patients D’∼10%, D’∼20% and D’∼30% were lower than DLiver in most, but not all patients (Supplementary Figure 1). In four patients (patient #1, #4, #5, #8), the D’∼10%, D’∼20% and D’∼30% were higher than the Dmean(Liver) suggesting no benefit from FTP.
In the FDGal PET/CT performed one month after treatment, we observed a generalized reduction in hepatic metabolic function of 17–30% after SBRT in two patients who had received high radiation dose to a relatively small volume of liver (V<15Gy 7.5 and 8.5%). An upregulation in hepatic metabolic function of 9–42% in the sub-volumes of non-irradiated liver was observed in eight patients who had received high radiation dose to a relatively large volume of liver (V<15Gy 18.6–46.5%). In four patients the hepatic metabolic function of non-irradiated liver was unchanged (change <5%). The relative spatial distribution of hepatic metabolic function in non-irradiated liver was visually evaluated. As shown in Supplementary Figure 2, the distribution did not change between the two FDGal PET/CT scans.
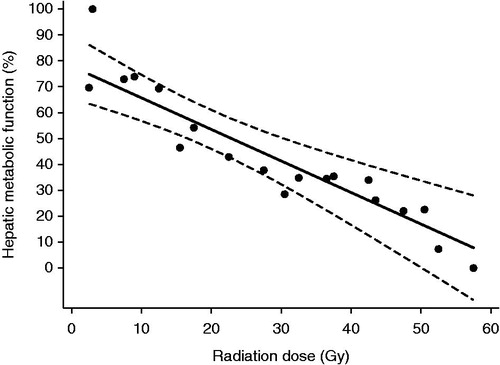
Altogether, a population-based analysis revealed a clear linear dose-response relationship between hepatic metabolic function and radiation dose developed from the FDGal PET/CT one month after treatment. The dose-response relationship () as well as a sigmoid-shaped fit of data (data not shown) did not support a threshold dose for reduction of hepatic metabolic function as the sigmoid-shaped fit of data were nearly linear. The decrease in hepatic function for each 10-Gy increment in dose (γ10Gy) was 12% (CI 95%: 7–17%). The dose resulting in 50% decrease in hepatic metabolic function (TD50) was 22.9 Gy in 3 fractions (CI 95%: 16.7–30.4 Gy).
Figure 2. Radiation dose-response relationship between hepatic metablic function (solid line) one month after FTP-SBRT delivered in three fractions as function of radiation dose with 95% confidence interval (dashed lines) and mean values in each dose interval (dots).
The post-SBRT reduction in hepatic metabolic function in sub-volumes of the liver with pretreatment high, intermediate and low hepatic metabolic function at the baseline did not differ. The slopes for high, intermediate and low hepatic metabolic function were −1.1 (CI 95%: −1.7 to −0.5), −1.0 (CI 95%: −1.7 to −0.4) and −0.5 (CI 95%: −1.2–0.1), respectively.
Discussion
The FTP-concept is relatively new and has so far only been sparsely studied [Citation10,Citation11]. Most studies are treatment planning comparisons of FTP and conventional planning strategies [Citation14,Citation18,Citation19]. There are only few clinical studies testing dose-painting to escalate the dose to the tumor based on functional imaging [Citation20,Citation21]. The present study is among the first clinical studies utilizing FTP with the aim to spare the best functioning normal tissue, in this case the liver. One study with a single patient treated with FTP for a liver tumor has been published demonstrating the possibility of minimizing the radiation dose to the 700 cc best functioning liver tissue by 1.6 Gy [Citation22].
FTP by use of FDGal PET/CT defined optimization sub-volumes was feasible without compromising the liver itself and other organs at risk and the dose to the best functioning sub-volumes of the liver was reduced by on average 2 Gy compared to the total liver volume (VLiver). This could have a clinical relevance for patients with hepatocellular carcinoma and cirrhosis and in patients with larger tumor volumes; it may expand the indication for SBRT in patients that cannot be offered standard SBRT for liver tumors due to the risk of RILD. Dose escalation to liver tumors could also be obtained if the sub-volumes of best functioning liver tissue is being spared to a minimal dose. Furthermore, it is possible that combined systemic therapy and SBRT may increase the risk of morbidity [Citation23] which increases the need for liver-sparing techniques in SBRT of liver tumors. Liver sparing may be achieved by FTP, proton therapy [Citation24] or both in combination [Citation19,Citation25]. The selection of patients for FTP is not yet clear, but in the present study it was not possible to spare V’∼10%, V’∼20% and V’∼30% in four patients. In these patients the target was localized in the cranial or caudal part of the liver surrounded by relative small volumes of liver tissue or adjacent to a RFA necrosis or the best functioning sub-volumes were close to the target and were exposed to a higher dose than the average liver. Potentially, the sub-volumes could have received an even higher dose if treated by conventional SBRT (Supplementary Figure 1).
With the limited number of patients, it was not possible to draw a firm conclusion on the morbidity of FTP-SBRT, but the treatment-related morbidity was low with no severe morbidities or RILD (Supplementary Table 1). The study indicates that sparing the best functioning liver is not on the expense of i.e., increased gastro-intestinal morbidity since the morbidity was not different from what is reported in larger cohorts of liver metastases patients treated with conventional SBRT [Citation8].
Data from the FDGal PET/CT performed one month after SBRT, does not support a threshold dose for reduction of the hepatic metabolic function indicating that it is necessary to spare as much as possible of the liver function. Many patients with liver metastases receive more local treatments over time and therefore it is also important to spare the liver function so patients can prolong the progression-free survival. It points at a linear decline in function of 12% per 10 Gy increase in dose and a TD50 of 22.9 Gy in 3 fractions (CI 95%: 16.7–30.4 Gy) (). The corrected BED from six to three fractions did not change the results. Howells et al. [Citation26] and Cao et al. [Citation27] both presented a linear dose-response relationship for liver tissue based on non-contrast CT scans and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), respectively. The studies indicated a threshold dose of 30-35 Gy and 17 Gy, respectively. However, CT and DCE-MRI does not quantify the metabolic function of the liver.
The change in hepatic metabolic function in sub-volumes of the liver characterized by high, intermediate and low baseline FDGal uptake did not differ significantly. This indicates a uniform dose-response relationship of the liver irrespectively to the baseline metabolic function. Furthermore, the relative spatial distribution of liver function remained unchanged at least over four weeks from treatment to follow-up scan in non-irradiated liver (Supplementary Figure 2). These two factors have important implications since the spatial redistribution of function over time and varying radio-sensitivity would be incompatible with or complicate the FTP strategy.
In non-irradiated liver there was a compensatory upregulation of hepatic metabolic function in patients who received radiation to large volumes of the liver and there was a downregulation of hepatic metabolic function in two patients receiving radiation to small volumes of the liver. Upregulation of liver function after radiotherapy reflects the observations done after SR of the liver [Citation28] and Cao et al. also found increased perfusion in non-irradiated liver tissue on DCE-CT after radiotherapy for liver tumors [Citation29]. The mechanism behind downregulation of liver function in the livers receiving radiation to a small volume is less clear. Radiation-induced cytokine response may explain the observed changes.
The most accepted guidelines for constraints to the liver in radiotherapy planning is the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) guideline based on normo-fractionated conformal radiation therapy and experience from liver resections [Citation9,Citation30]. There is a need for methods proving a more precise assessment of the regional liver function as well as strategies to spare the best functioning parts of the liver. The present study is one of the first suggestions of quantifications of the hepatocyte function and FTP to minimize the radiation dose to the normal surrounding tissue.
The present study is pioneering the assessment of liver function for FTP-SBRT of liver tumors. There are, however, limitations of the method that needs further elaboration. The deformable co-registration of the planning CT and the FDGal PET/CT is crucial in FTP and mismatch alignment of imaging series and motion artifacts during acquisition of the PET scans are sources of errors. Due to motion artifacts, sub-volumes of best functioning liver tissue may not be identified and will not be spared. Increased resolution by gated PET and treatment under breath-hold or gating may be the ways to further improvement of the technique.
In conclusion, the present study demonstrates the feasibility and safety of FTP of liver metastases and it was possible to reduce the dose to the sub-volumes of best functioning liver tissue by on average 2 Gy. A TD50 of 22.9 Gy in 3 fractions and a γ10Gy of 12% were found in liver without parenchymal liver disease. Investigation of FTP in patients with cirrhosis and hepatocellular carcinoma is the next step. Functional imaging of the liver can be utilized in FTP-SBRT with potential important liver sparing.
IONC_A_1366051_Supplementary_Information.docx
Download MS Word (1.1 MB)Acknowledgments
The authors thank research nurse Annette Schouboe for assistance with the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andres A, Majno PE, Morel P, et al. Improved long-term outcome of surgery for advanced colorectal liver metastases: reasons and implications for management on the basis of a severity score. Ann Surg Oncol. 2008;15:134–143.
- Donadon M, Solbiati L, Dawson L, et al. Hepatocellular carcinoma: the role of interventional oncology. Liver Cancer. 2016;6:34–43.
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917.
- Hoyer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys. 2012;82:1047–1057.
- Mendez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase i-ii study. Acta Oncol. 2006;45:831–837.
- Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830.
- Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28–e37.
- Fode MM, Hoyer M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol. 2015;114:155–160.
- Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94–S100.
- Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47:551–560.
- Partridge M, Yamamoto T, Grau C, et al. Imaging of normal lung, liver and parotid gland function for radiotherapy. Acta Oncol. 2010;49:997–1011.
- Sorensen M, Munk OL, Mortensen FV, et al. Hepatic uptake and metabolism of galactose can be quantified in vivo by 2-[18F]fluoro-2-deoxygalactose positron emission tomography. Am J Physiol Gastrointest Liver Physiol. 2008;295:G27–G36.
- Sorensen M, Mikkelsen KS, Frisch K, et al. Hepatic galactose metabolism quantified in humans using 2-18F-fluoro-2-deoxy-d-galactose PET/CT. J Nucl Med. 2011;52:1566–1572.
- Fode MM, Petersen JB, Sørensen M, et al. 18F]fluoro-2-deoxy-d-galactose positron emission tomography guided functional treatment planning of stereotactic body radiotherapy of liver tumours. Phys Imag Radiat Oncol. 2017;1:28–33.
- Poulsen PR, Worm ES, Hansen R, et al. Respiratory gating based on internal electromagnetic motion monitoring during stereotactic liver radiation therapy: first results. Acta Oncol. 2015;54:1445–1452.
- Buus S, Grau C, Munk OL, et al. Individual radiation response of parotid glands investigated by dynamic 11C-methionine PET. Radiother Oncol. 2006;78:262–269.
- Kirkwood B, Sterne J. Medical statistics. Malden (MA): Blackwell Science; 2007.
- Lavrenkov K, Christian JA, Partridge M, et al. A potential to reduce pulmonary toxicity: the use of perfusion SPECT with IMRT for functional lung avoidance in radiotherapy of non-small cell lung cancer. Radiother Oncol. 2007;83:156–162.
- Bowen SR, Saini J, Chapman TR, et al. Differential hepatic avoidance radiation therapy: proof of concept in hepatocellular carcinoma patients. Radiother Oncol. 2015;115:203–210.
- Duprez F, De Neve W, De Gersem W, et al. Adaptive dose painting by numbers for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:1045–1055.
- Rasmussen JH, Hakansson K, Vogelius IR, et al. Phase I trial of 18F-Fludeoxyglucose based radiation dose painting with concomitant cisplatin in head and neck cancer. Radiother Oncol. 2016;120:76–80.
- De Bari B, Jumeau R, Deantonio L, et al. Role of functional imaging in treatment plan optimization of stereotactic body radiation therapy for liver cancer. Tumori. 2016;102:e21?e24.
- Brade AM, Ng S, Brierley J, et al. Phase 1 Trial of Sorafenib and stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2016;94:580–587.
- Petersen JB, Lassen Y, Hansen AT, et al. Normal liver tissue sparing by intensity-modulated proton stereotactic body radiotherapy for solitary liver tumours. Acta Oncol. 2011;50:823–828.
- Petersen JB, Fode MM, Worm ES, et al. FDGal guided functional treatment planning for liver SBRT using scanning proton beams. Abstract PTCOG. PTCOG55, O 69, 2016.
- Howells CC, Stinauer MA, Diot Q, et al. Normal liver tissue density dose response in patients treated with stereotactic body radiation therapy for liver metastases. Int J Radiat Oncol Biol Phys. 2012;84:e441–e446.
- Cao Y, Wang H, Johnson TD, et al. Prediction of liver function by using magnetic resonance-based portal venous perfusion imaging. Int J Radiat Oncol Biol Phys. 2013;85:258–263.
- Kwon YJ, Lee KG, Choi D. Clinical implications of advances in liver regeneration. Clin Mol Hepatol. 2015;21:7–13.
- Cao Y, Pan C, Balter JM, et al. Liver function after irradiation based on computed tomographic portal vein perfusion imaging. Int J Radiat Oncol Biol Phys. 2008;70:154–160.
- Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821.
