Abstract
Background: Little information on the natural history of patients with localized NSCLC is available since many of the studies covering the subject lack information on pathological confirmation, staging procedures and comorbidity. No randomized studies have compared SBRT with no treatment for patients with localized NSCLC. The purpose of this study was to evaluate whether SBRT has influence on overall survival for patients with localized NSCLC and investigate the effect of baseline ventilatory lung function on overall survival.
Material and methods: From 2007 to 2013, 136 patients treated with SBRT at Odense University Hospital were prospectively recorded. The thoracic SBRT consisted of three fractions of 15–22 Gy delivered in 9 days. For comparison, a national group of 73 untreated patients in the same time period was extracted from the Danish Lung Cancer Registry. All patients had histologically/cytologically proven NSCLC T1-2N0M0 with a tumour diameter ≤5 cm.
Results: The 5-year relative survival was 44% versus 7% for the SBRT and untreated groups, respectively. In a propensity score matched comparison the median overall survival was 47 months versus 11 months for the SBRT and untreated groups, respectively (p < .05). On multivariate analysis, SBRT was significantly associated with improved prognosis while ECOG performance status 2+ and tumour diameter ≥3 cm significantly predicted poorer prognosis. Severe to very severe reduction of forced expiratory volume in one second (FEV1) did not predict poorer survival for the SBRT treated patients with localized NSCLC.
Conclusions: SBRT offers more favourable survival than no treatment for patients with localized NSCLC. Performance status of 0–1, tumour diameter less than 3 cm and SBRT predicted improved survival. SBRT should not be withheld for patients with localized NSCLC based on poor ventilatory lung function.
Introduction
Lung cancer is the leading cause of cancer-related death among both men and women [Citation1]. Despite the poor prognosis of lung cancer some patients may be cured if the disease is diagnosed in an early stage. Lobectomy remains the cornerstone of treatment for patients with localized non-small cell lung cancer (NSCLC) [Citation2,Citation3]. However, some patients refuse surgery or are medically inoperable due to comorbidities, advanced age or poor performance status. According to guidelines stereotactic body radiation therapy (SBRT) is recommended for this group of patients [Citation4,Citation5].
Several investigators have reported excellent local control rates with SBRT similar to those seen with surgery [Citation6–8]. Before the introduction of SBRT, conventional radiotherapy (convRT) was used, but many patients were not fit for a schedule of radiotherapy using many fractions. SBRT to patients with localized NSCLC gave similar local control rates and survival compared with high dose convRT in a retrospective study [Citation9]. The randomized phase II SPACE study compared SBRT (66 Gy in three fractions) with convRT (70 Gy in 35 fractions) and no difference in survival was found [Citation10]. Only limited data of untreated patients with localized NSCLC are available. Many of the previously published studies on the natural history lack information on pathological confirmation, staging procedures and comorbidity. These studies have demonstrated poor overall survival [Citation11–13], but no randomized study of SBRT versus no treatment has been conducted. Although SBRT offers excellent local tumour control, it remains unclear if all patients with localized NSCLC benefit from the treatment.
Up to 75% of all patients treated with SBRT for a localized NSCLC had spirometry results suggesting chronic obstructive lung disease (COPD), and COPD is the most common reason for medically inoperability [Citation14]. COPD itself has severe prognosis, and the survival for COPD patients declines with poorer ventilatory capacity. The impact on survival for patients treated with SBRT for localized NSCLC with poor ventilatory capacity has not been fully investigated.
The present retrospective study was conducted to investigate the survival of patients with localized NSCLC treated with SBRT compared with patients who received no treatment for the lung cancer. Additionally, the study investigates the effect of baseline ventilatory lung function estimated by forced expiratory volume in 1 s (FEV1) on overall survival in patients with localized NSCLC.
Material and methods
The analysis was constructed as a descriptive observational study. Two cohorts of patients with localized NSCLC were established. All patients were diagnosed with histologically or cytologically proven T1-2N0M0 NSCLC with a tumour diameter of maximum 5 cm.
Setting
Data of one cohort (SBRT group) were obtained from consecutive patients with localized NSCLC treated with SBRT at the Department of Oncology at Odense University Hospital, Denmark. The patients were referred from the Region of Southern Denmark and the Region of Zealand for treatment with SBRT. A national control group of untreated patients (NoTx group) was extracted from the Danish Lung Cancer Registry (DLCR). The DLCR database was established in 2000 and contains information on patients’ demographic, TNM stage, histology, FEV1, ECOG performance status (PS), Charlson Comorbidity Index (CCI), diagnostic procedures and total pack years of tobacco consumption [Citation15].
All patients were diagnosed with localized NSCLC in the period of January 2007–December 2013. According to the 6th edition of TNM Classification of malignant tumours, patients in the NoTx group with tumour diameter >5 cm could have been classified as T2 [Citation16]. The charts from patients diagnosed prior to 2010 with T2 tumours were reviewed to exclude patients with tumour sizes exceeding 5 cm. Additional data on tumour measures, if needed, were obtained by contacting the local department of respiratory medicine (DRM) that diagnosed the patients. Patients were excluded from further analyses in this study if no reply at the date of analyses.
Ventilatory lung function
Only FEV1 was available for analyses. FEV1 values in DLCR database were reported from the local DRM. For the SBRT group, the FEV1 values were recorded at the first fraction of SBRT. The predicted values of FEV1 (FEV1%pred) were calculated from the Global Lung Function Initiative reference equations [Citation17]. To facilitate interpretation of the data, the FEV1 was graded 1–5 as proposed by Quanjer et al. [Citation18] for patients with bronchial obstruction. The FEV1 z-score defined by the lower limit of normality is signifying the number of standard deviations from the mean predicted value of FEV1 and is unbiased by sex, age, height and ethnic groups. Grade 1, none or mild reduction; z-score ≥ −2, grade 2, moderate reduction; z-score ≥ −2.5 to < −2, grade 3, moderately severe reduction; z-score ≥ −3 to < −2.5, grade 4, severe reduction; z-score ≥ −4 to < −3 and grade 5, very severe reduction; z-score < −4.
Patients
Data from the SBRT group were obtained from patients’ charts and radiation therapy plans. The pretreatment evaluation for patients treated with SBRT included complete clinical examination incl. ECOG PS, chest X-ray, CT scan of the chest and upper abdomen and measurement of lung function. The tumour diameter was obtained from the radiation therapy plan.
A review of records identified 136 patients with localized NSCLC treated with SBRT. Eighty-nine patients were considered medically inoperable, 41 patients were considered best treated by SBRT due to age, frailty or comorbidity and six patients denied surgery. All were treated with SBRT as described previously [Citation9]. In summary, SBRT consisted of three fractions of radiotherapy. Prior to October 2008, the gross tumour volume (GTV) was treated to a total central dose of 45 Gy and the planning target volume (PTV) treated to 30 Gy. After October 2008, the GTV was treated to 66 Gy and the PTV treated to 45 Gy. The treatment duration was maximum 9 days.
For the national NoTx group, 126 untreated patients with localized NSCLC in stage T1–T2N0M0 diagnosed from 2007 to 2013 were identified in DLCR. The tumour diameter was obtained for the patients’ charts or by contacting the local DRM. Of these, eight had tumour diameter >5 cm and nine patients had unreported tumour diameter. One hundred and nine patients thus remained in the cohort of untreated patients. To exclude patients ineligible for SBRT, 36 patients who survived less than one month after establishing the diagnosis of NSCLC, were excluded leaving the NoTx group consisting of 73 patients for further analyses. The patients in the NoTx group did not receive any surgery, antineoplastic agents or radiotherapy. The untreated patients were not necessarily considered medically inoperable. Twenty-five patients denied any kind of treatment, eight patients received observation management only and 14 patients were too frail to receive treatment. Four patients had concurrent cancer and were not considered for treatment for the localized NSCLC, and for 22 patients the reason for missing referral to the department of oncology to receive SBRT was not stated.
Information on comorbidity using the Danish National Patient Registry containing data on all intervention related to diagnostic evaluation and treatment for somatic patient admission in Denmark was extracted from DLCR for both groups. Lung cancer diagnosis was excluded from the scoring. The classification of comorbidity was done according to CCI. Patients were grouped as 0–1, 2–3 or 4+ comorbid conditions.
Follow-up
Follow-up was performed for patients in the SBRT group 5 weeks after treatment, then every third months in a 2-year period and finally in 6-month intervals the next 3 years. The follow-up included medical history, clinical examination, chest X-ray (standard prior to 2010) or CT-scan of chest and upper abdomen (after 2010) and measurement of lung function. The patients in the NoTx group did not have a planned follow-up programme. However, some patients attended regular visit at the local DRM to detect and palliate symptoms of the lung cancer, for example, dyspnoea and pain.
Statistical methods
The primary endpoint of the study was overall survival (OS). The survival rates were calculated from the date of malignant pathology diagnosis. OS was defined as the time to death from any cause including lung cancer. The OS of the study population to that of the general Danish population by means of methods from relative survival (RS) was conducted. The survival outcomes of the study populations were analysed for men and women separately in comparison with the expected survival of the general population in which each patient was matched to a control with the same sex and age in the year of NSCLC diagnosis. Population tables from the Danish Statistics (www.statistikbanken.dk/HISB9) were used. All medical documentation for both groups was reviewed for survival status. All data were analysed 1st of June 2017. Univariate analyses were used to compare the two groups. Means/medians were compared using T-test/Mann–Whitney test. Group proportions were compared using Fisher’s exact test. Multivariable analyses used Cox regression to explore if any of the variables influenced overall survival. Propensity score matching (PSM) was performed to reduce confounding between the groups. The PSM was done utilizing the nearest-neighbour methodology without replacement. The survival analyses were calculated using the Kaplan–Meier method for both the unmatched and matched comparisons of patients. The log rank test was used for testing differences in survival rates. Secondary PSM analyses stratified by FEV1 reduction grade into to two groups (FEV1 reduction grade 1–3 versus FEV1 reduction grade 4–5) were performed. In all the analyses, a two-tailed p value of <0.05 was considered to be statistically significant.
Results
In the SBRT group 136 patients were enrolled and 73 patients in the NoTx group. The median potential follow-up time was 70.1 months (41–123 months) and 84.4 months (41–124 months) for the SBRT and NoTx groups, respectively. Patients in the NoTx group were older (p < .001), had fewer PET/CT scans performed as a staging procedure (p < .001) and were less likely to have been diagnosed with adenocarcinoma (p = .004). Median FEV1 and FEV1 z-score was 1.42 versus 1.42 L and −2.47 versus −2.13 in the SBRT and NoTx groups, respectively. Further details of baseline characteristics are described in . In the study population, the fraction of patients who received SBRT increased from 52% in 2007–2009, to 57% in 2010–2011, and 80% in 2012–2013.
Table 1. Patients’ characteristics.
As illustrated in the median OS (mOS) was 47.8 versus 11.6 months for the SBRT and NoTx groups, respectively (p < .001). The observed 1-year OS was 87% versus 48% and the 5-year OS was 35% versus 5% in the SBRT and NoTx groups, respectively. No difference in mOS was observed in the NoTx group between patients with or without PET/CT as a staging procedure; 12.5 versus 9.8 months, respectively (p = .12). Subgroup analysis for the NoTx group was performed according to the five different reasons for patients not receiving treatment and there was no statistically significant difference in mOS; for example, 12.8 months for patients who refused treatment and 10.1 months for patients where reason for missing referral was not stated. (p = .65). The expected 1-year survival and 5-year survival were 96% versus 94% and 80% versus 70% for the SBRT and NoTx groups, respectively. At 1 year and 5 years after NSCLC diagnosis the RS was 90% versus 51% and 44% versus 7% for the SBRT and NoTx groups, respectively. A multivariable Cox regression analysis of overall survival for the total study population indicated that pack years, adenocarcinoma, FEV1 reduction grade, sex and CCI did not statistical significantly influence survival. SBRT was a significant factor and associated with improved prognosis with a hazard ratio (HR) = 0.25 (95% CI: 0.17–0.35), while ECOG performance status 2+ with HR =1.64 (95% CI: 1.18–2.27) and tumour diameter >3 cm with HR =1.72 (95% CI: 1.24–2.38) also were significant factors but associated with poorer prognosis (). The diagnostic procedure of a PET/CT was associated with improved prognosis in univariate analysis, but was not found to be a significant factor in multivariate analysis.
Figure 1. Kaplan–Meier survival curves of overall survival for the SBRT group and the NoTx group before PSM.
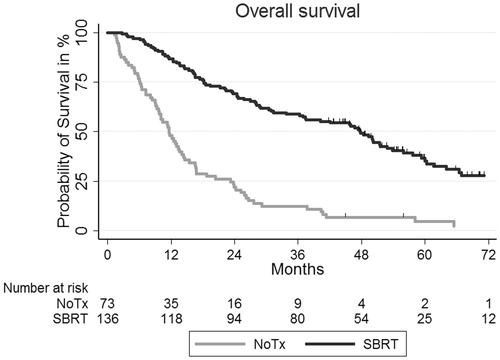
Table 2. Proportional hazards model for mortality among the total study population in univariate and multivariate models.
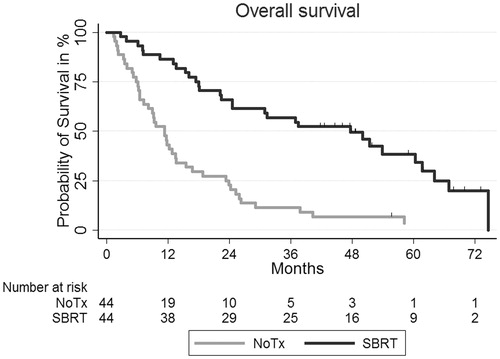
PSM identified 44 patients from each group. Ten insufficient matches were observed. The groups were matched on three clinical co-variables known to be related to overall survival: age, FEV1 z-score and sex. Baseline characteristics after PSM in the two groups were well balanced; mean age of 76.3 versus 76.1 years, FEV1 z-score of −2.23 versus −2.34, and 22 versus 22 women, the SBRT and NoTx groups, respectively. In the matched comparison no statistically significant differences in tobacco consumption, CCI and tumour size were observed; 41 versus 41 packyears (p = .88), 2 versus 2 (p = .91) and 3.1 versus 3.0 cm (p = .71) in the SBRT and NoTx groups, respectively. However, more patients in the SBRT group had adenocarcinomas 23 versus 14 patients in the SBRT and NoTx group, respectively (p = .04). As illustrated in , there was a statistically significant difference in mOS in the matched comparison, 47 versus 11 months in the SBRT and NoTx groups, respectively (p < .001). Secondary analyses showed that the survival for patients with FEV1 reduction grade 1–3 was affected significantly by SBRT treatment with HR =0.39 (95% CI: 0.22–0.68) after PSM. There was also a statistically significant difference in survival for patients with FEV1 reduction grade 4–5 with SBRT compared with the NoTx group with a HR =0.16 (95% CI: 0.05–0.49) after PSM.
Figure 2. Kaplan–Meier survival curves of overall survival for the SBRT group and the NoTx group after PSM.
According to information available in medical records none of the patients treated with SBRT experienced acute grade 3+ toxicity or SBRT related death. The most common side effects were skin rash, rib fracture, cough and radiological pneumonitis/fibrosis without clinical symptoms. Fifty-one patients had recurrence of NSCLC after treatment with SBRT. Of these, 21 patients did not receive any salvage treatment, 22 patients received palliative treatment (RT, chemotherapy or tyrosine kinase inhibitor) and eight patients received curative treatment (SBRT, chemo-radiotherapy or lobectomy). There was no statistically significant difference in mOS for patients without and with treatment, 21.3 versus 37.4 months respectively (p = .40).
Discussion
The current data set represents a large single-institutional cohort of patients with localized NSCLC treated with SBRT compared with a national cohort extracted from DLCR of patient with localized NSCLC without treatment. In summary, the survival was more favourable among patients treated with SBRT compared with patients who did not receive any treatment. In total, there was a 75% lower mortality rate among patients with localized NSCLC treated with SBRT compared with patients without treatment. The median overall survival was approximately 3 years longer for SBRT treated patients and the relative survival at 5 years was 44% for the SBRT group and 7% for the NoTx group. Severe to very severe reduction of FEV1 (FEV1 reduction grade 4–5) did not predict for poorer survival for SBRT treated patients with localized NSCLC compared to patients with FEV1 reduction grade 1–3.
Wao et al. conducted a systematic review of the natural history of patients with confirmed diagnosis of lung cancer without active treatment and found that patients with localized NSCLC had a median OS of 11.9 months [Citation19]. Many of the included studies in this review were not analysed in the era of SBRT and hence comparison to patients treated with SBRT can be difficult. However, the present study compared survival in localized NSCLC patients with or without SBRT in the same time period and found similar poor OS for untreated patients. In most of the previous studies comparing SBRT with no treatment, staging procedures were not stated [Citation11–13,Citation19–21]. Some studies were conducted in the PET era, but it was not stated how many patients had PET or mediastinal staging performed. In the present study, full information on staging procedures was available in both groups of patients with cytologically/histologically proven localized NSCLC. Fewer patients had PET/CT performed in the NoTx group (64%), but it did not affect the overall survival for the untreated patients. Some of the studies on natural history of patients with localized lung cancer had no information on treatment to specific symptoms with, for example, palliative radiotherapy. In the present study, the patients in the NoTx group did not receive any kind of oncologic treatment including palliative treatment. The present study also took changes in IASLC TNM Classification into consideration. Any misclassification in both groups is thus considered to be slight.
It is a weakness of the study that the reason for not being referred to treatment is not known for all of the untreated patients. One-third of the patients in the untreated group refused treatment. However, this study showed that patients in the NoTx group had the same prognosis regardless of lacking information of no referral to treatment or the patients refused treatment. Patients in the untreated group were not necessarily evaluated for surgery. Thus, these patients may have been in better medical condition than those who were treated with SBRT and the median overall survival may be overestimated. The propensity score matching was conducted in an attempt to control for unrecognized confounders. However, unbalances may have persisted in the nature of a retrospective study. Shirvani et al. also performed propensity score matching in elderly patients with localized NSCLC and found no statistically significant difference in overall survival in patients treated with lobectomy and SBRT, and that untreated patients had poor outcome [Citation22]. In a previous study on localized NSCLC there was no statistically significant difference between a Charlson Comorbidity Index score in SBRT and untreated patients, and that patients with higher Charlson Comorbidity Index score still benefit for SBRT suggesting that lung cancer is the main driver for death in this population [Citation21]. The same was observed in the present study with no differences in CCI between the NoTx and SBRT groups. Evaluating 5-year survival using relative survival as a measure was therefore advantageous compared with disease-free survival and overall survival because it accounted for competing causes of death like comorbidity by adjusting for the general survival rate for that same age and date of diagnosis. Often, the reported causes of death in population based studies are not consistent. Given the median age of NSCLC diagnosis (74 years) and the competing risk of death from comorbidities, relative survival may be a better measure of survival since it is not required to know the cause of death for calculation. The relative 5-year survival of 44% and 7% for the SBRT and untreated groups provides valuable information for the patients and the clinicians. The relative survival in the SBRT group being much less than 100% is probably partly due to tumour control is not obtained in all patients. However, patients diagnosed with smoking related cancers may also experience excess mortality compared to the general population due to other smoking related conditions.
In a study of Henderson et al. baseline pulmonary function in terms of low FEV1 did not predict for decreased survival for patients with localized NSCLC treated with SBRT. However, an increase in baseline FEV1 predicted three times higher risk of death after treatment [Citation23]. Stephans et al. also found that lower baseline FEV1%pred had better survival than the total study group [Citation24]. In the latter study, height, age and gender were also included in order to reduce potential confounding. Both studies found that patients considered medically inoperable who had the highest baseline FEV1 often suffered and died from cardiac comorbid conditions. In the present study, FEV1 reduction grades did not have statistically significant influence on OS. However, the patients with FEV1 reduction grade 4–5 did benefit more from SBRT compared to patients with FEV1 reduction grade 1–3. The Cox regression analyses have been repeated with FEV1%pred as a continuous variable and FEV1-reduction still did not have influence on overall survival (data not shown). These results may indicate that other comorbid conditions than COPD may account for the survival difference of SBRT treated patients with localized NSCLC. Klement et al. found that CCI did not predict the risk of early death and all patients should be offered SBRT despite of comorbid conditions [Citation25]. Klement et al. also found that ECOG PS was significantly associated with early death and suggested that a possible solution for longer overall survival could be a comprehensive geriatric assessment (CGA). The relative survival for both groups of patients in this study suggests that not only lung cancer death is the main driver for mortality, but may also be explained by the frailty of both groups. Therefore, a CGA might improve overall survival.
In conclusion, there was a significant difference in overall survival favouring SBRT to no treatment in patients with localized NSCLC. Performance status of 0–1, tumour size less than 3 cm in diameter and SBRT predicted improved prognosis. SBRT should not be withheld for patients with localized NSCLC based on low baseline FEV1%pred.
Acknowledgments
The study was made as part of AgeCare (Academy of Geriatric Cancer Research), an international research collaboration based at Odense University Hospital, Denmark. We acknowledge the Danish Lung Cancer Registry for providing data to this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S.
- Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol. 2014;25:1462–1474.
- Guckenberger M, Allgauer M, Appold S, et al. Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol. 2013;8:1050–1058.
- Ricardi U, Badellino S, Filippi AR. Stereotactic radiotherapy for early stage non-small cell lung cancer. Radiat Oncol J. 2015;33:57–65.
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–3296.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076.
- Mokhles S, Verstegen N, Maat APWM, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: results from propensity score analysis. Lung Cancer. 2015;87:283–289.
- Jeppesen SS, Schytte T, Jensen HR, et al. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol. 2013;52:1552–1558.
- Nyman J, Hallqvist A, Lund JS, et al. SPACE – a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121:1–8.
- McGarry RC, Song G, des Rosiers P, et al. Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest. 2002;121:1155–1158.
- Chadha A. Survival in untreated early stage non-small cell lung cancer. Anticancer Res. 2005;25:3517–3520.
- Vrdoljak E, Miše K, Sapunar D, et al. Survival analysis of untreated patients with non-small-cell lung cancer. Chest. 1994;106:1797–1800.
- Jeppesen SS, Hansen NC, Schytte T, et al. Comparison of survival of chronic obstructive pulmonary disease patients with or without a localized non-small cell lung cancer. Lung Cancer. 2016;100:90–95.
- Jakobsen E, Green A, Oesterlind K, et al. Nationwide quality improvement in lung cancer care: the role of the Danish Lung Cancer Group and Registry. J Thorac Oncol. 2013;8:1238–1247.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714.
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343.
- Quanjer PH, Pretto JJ, Brazzale DJ, et al. Grading the severity of airways obstruction: new wine in new bottles. Eur Respir J. 2014;43:505–512.
- Wao H, Mhaskar R, Kumar A, et al. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev. 2013;2:10.
- Haasbeek CJ, Palma D, Visser O, et al. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol. 2012;23:2743–2747.
- Nanda RH, Liu Y, Gillespie TW, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: a National Cancer Data Base analysis. Cancer. 2015;121:4222–4230.
- Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys. 2012;84:1060–1070.
- Henderson M, McGarry R, Yiannoutsos C, et al. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:404–409.
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol. 2009;4:838–844.
- Klement RJ, Belderbos J, Grills I, et al. Prediction of early death in patients with early-stage NSCLC – can we select patients without a potential benefit of SBRT as a curative treatment approach? J Thorac Oncol. 2016;11:1132–1139.
