Abstract
Purpose: Inadequate clinical target volume (CTV) definition is likely to be a major contributing factor to local recurrence (LR) rate after radiotherapy. Our aims were to identify sites of prostate cancer LR in biochemical recurrence post-prostatectomy using 18F-Fluorocholine (18F-FCH) positron emission tomography/computed tomography (PET/CT) and to compare different CTV-delineation guidelines in a cohort of postoperative patients.
Material and methods: Thirty-six patients presenting with LR within the prostatic bed on 18F-FCH PET/CT between 10/2011 and 06/2016 were included in this retrospective study. Median PSA at the time of 18F-FCH PET/CT was 2.7 ng/mL (0.8–9.4) and median PSA doubling time was 11 months (3–28). For each patient, the CTVRTOG, CTVFROGG and CTVEORTC following the corresponding guidelines were outlined and compared. Forty-one LR were delineated using a gradient-based method and the percentage of FCH uptake included in each CTV was evaluated.
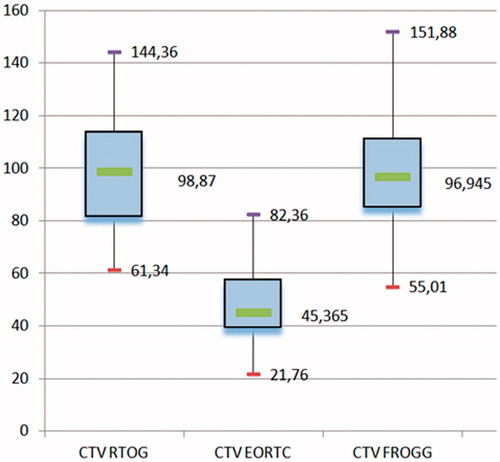
Results: The anastomosis was the most common recurrence site (52.8%), followed by the retrovesical region (31.7%) and the bladder neck (7%). The median SUV max value was 4.8 (2.3–16.1). The percentage of LR entirely included in the CTVRTOG was not significantly different from that included in the CTVFROGG (84% versus 83%, p = .5). Significantly more recurrences were included in the CTVRTOG volume compared to the CTVEORTC (84% versus 68%, p=.006), due to a better coverage of the bladder neck and retrovesical regions. Six out of 10 relapses occurring in the posterior region of the anastomosis were not covered by any of the CTVs.
Conclusions: In our study, the CTVRTOG and CTVFROGG ensured the best coverage of LR seen on 18F-FCH PET/CT. When outlining the prostatic fossa, greater coverage of the posterior vesico-urethral region may allow better coverage of potential microscopic disease.
Introduction
Radical prostatectomy (RP) with or without pelvic lymph node dissection is one of the main curative options for prostate cancer (PCa). However, approximately 20–30% patients subsequently experience a biochemical recurrence (BR) [Citation1], defined as a rise in prostate specific antigen (PSA) with two sequential values ≥0.2 ng/mL [Citation2]. For patients with adverse pathological features like high Gleason score, lymphovascular invasion, extracapsular extension, or positive surgical margins, the recurrence rate can reach as high as 40% [Citation3]. The standard treatment for most patients with BR after RP is salvage radiotherapy (SRT) but the timing of SRT, total dose, benefit of combination with androgen deprivation therapy (ADT) or for pelvic lymph node irradiation remain a matter of debate. Furthermore, despite adjuvant radiotherapy (ART) after surgery, 40% of patients will ultimately develop a local recurrence (LR), highlighting the importance of an adequate radiation dose and definition of the clinical target volume (CTV). However, no consensus has yet been reached on the CTV definition, and to date, several different guidelines have been published to address the need for standardization of postoperative target delineation, including the Radiation Therapy Oncology Group (RTOG), the European Organisation for Research and Treatment of Cancer (EORTC) and the Faculty of Radiation Oncology Genito-Urinary Group (FROGG) consensus guidelines [Citation4–8].
Modern imaging such as 18F-Fluorocholine (18F-FCH) positron emission tomography/computed tomography (PET/CT) has been used increasingly in the setting of PCa during the last years, with preliminary promising studies showing sensitivities as high as 93% and specificities of 76% [Citation9]. More recently, despite the lack of large validation trials, 18F-FCH PET/CT is being used increasingly to assist radiation oncologists in targeting nodal or oligometastatic disease in patients with relapsed PCa. Detection of focal prostatic recurrence is however more challenging as 18F-FCH PET/CT suffers from a low spatial resolution with inconsistent sensitivity in this setting, ranging from 64 to 100% [Citation10]. For now, magnetic resonance imaging (MRI) remains the modality chosen in this indication [Citation11].
The present study aims to describe the pattern of local relapse as diagnosed on 18F-FCH PET/CT in patients with BR after RP, to compare the three main delineation consensus guidelines (EORTC, RTOGG and FROGG), and to evaluate the potential geographic miss depending on the guideline used.
Material and methods
Patient population
One hundred and thirty-six patients with BR following RP for prostate adenocarcinoma underwent a 18F-FCH PET/CT from October 2011 to April 2016 at our institution. None of the patients received ADT or showed any clinical evidence of distant metastases. FCH PET/CT was performed in patients with a PSA value >1 ng/mL or with a lower level but a doubling time <6 months. Among the 136 patients, 36 experienced local relapse only constituting the final cohort of this study. Median age at time of surgery was 71.3 years (54.5–82.2) and BR occurred after a median postsurgical interval of 85 months (3–132). Median PSA at the time FCH PET/CT was performed was 2.7 ng/mL (0.8–9.4) and median PSA doubling time 11 months (3–28). Primary disease and characteristics of relapses are shown in . All patients gave permission for the use of their clinical data for scientific purposes and informed consent for the anonymous publication of data.
Table 1. Patients and disease characteristics.
Imaging procedure
PET-CT was performed using Biograph mCT S64 (Siemens Medical, Erlangen, Germany). Patients fasted for at least 6 h before intravenous injection of 3–4 MBq/kg of 18F-FCH (IASOcholine, Graz-Seiersberg, Austria). Dynamic PET/CT imaging of the pelvis was first performed with a low-dose CT from 0 to 10 min after injection. After a period of approximately 60 min when the patients remained in a quiet room, whole-body PET/CT (from mid-forehead to mid-thigh) was performed within 2 min per bed position. CT was done with normal shallow respiration using a low-dose setting (120 kv, 80 mAs). Intravenous iodinated contrast was given without contra-indication. Data obtained from the CT scan were used for attenuation correction of PET data and for fusion with attenuation-corrected PET images.
FCH PET/CT interpretation
All 18F-FCH PET/CT images were analyzed with the dedicated software (Syngo.via, Siemens AG, Erlangen, Germany), which allowed the review of PET, CT and fused imaging data. Local relapse was defined as recurrent or persistent disease involving the prostatic fossa on PET/CT. The interpretation of 18F-FCH PET/CT scans was done by a board-certified nuclear medicine physician (PR) who remained blinded for the CTV outlines. PET/CT was interpreted as positive for malignant disease on the basis of foci of non-physiological 18F-FCH uptake in the prostatic bed higher than the surrounding background, not attributable to physiologic elimination, with no predetermined cutoff maximum standard uptake (SUVmax) value.
Target volumes definition
Different target volumes were outlined on FCH PET/CT examination using The MIM® software (6.5.2, MIM Software Inc., Cleveland, OH) in each patient.
First, three different CTVs of LR named CTVRTOG CTVEORTC and CTVFROGG were outlined manually on the contrast enhanced CT of the PET/CT following the guidelines published by RTOG [Citation4], the EORTC [Citation5] and the FROGG [Citation8], respectively. All contours were done by the same operator (OEK) based on the information from the above papers. This operator remained blinded for the PET images. Particular consideration was given to pathology reports, especially regarding seminal vesicle invasion. Where the RTOG guidelines specified a range of CTVs limits, the largest boundaries were considered. Organs at risk (OARs) including rectum, bladder, vesico-urethral anastomosis and penile bulb were contoured on axial CT slices and served as anatomical landmarks for CTV delineation. The entire bladder was outlined from the vesico-urethral anastomosis to its apex and the rectum from the lower part of the ischial tuberosity to the recto-sigmoid junction. All outlines were reviewed by a senior oncologist specializing in genitourinary (GU) malignancy.
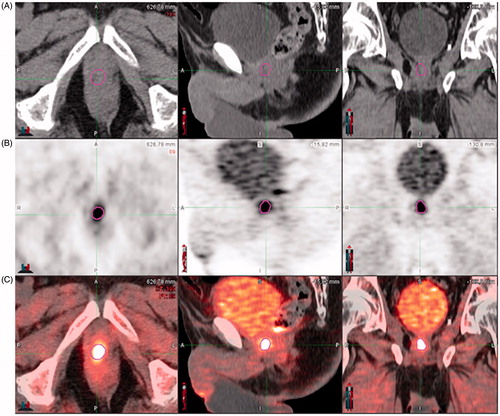
Second, a biological target volume named BTVRELAPSE was outlined around the local site of recurrence on the PET dataset using a gradient-based algorithm [Citation12] (PET-Edge tool), relying on the change in intensity/activity at the tumor borders (). The location of the BTVRELAPSE was documented. Overlap between BTVRELAPSE and CTVRTOG, CTVEORTC, CTVFROGG, respectively, was visually assessed for each patient. BTVRELAPSE was considered as ‘included’, ‘outside’ or ‘partially included’.
Figure 1. Biological target volume (BTVRELAPSE) located on the vesico-urethral anastomosis and outlined using the gradient-based algorithm (PET-Edge) provided by MIM-Vista® (6.5.2, MIM Software Inc., Cleveland, USA). (A) CT image. (B) 18F-FCH PET images. (C) Registration.
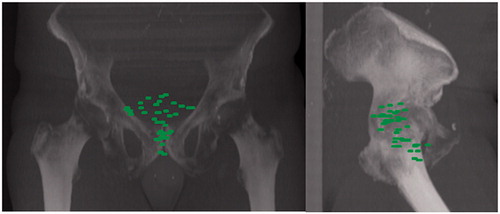
In order to get a mapping of the LRs in the lower pelvis, all 18F-FCH PET sets were registered with a single reference scan (taken from a PCa patient) using automatic deformable registration using the ATLAS option of the MIMVista® 6.5.2 Software.
Statistics
Descriptive statistics were used to characterize the study cohort. Continuous variables were summarized as median (range). Categorical variables were presented as counts and percentages. Statistical analysis using the Mann–Whitney test was performed with the software package MATLAB™ 7.8 (The MathWorks©, Natick, MA, USA). p Values less than .05 were considered to indicate statistical significance.
Results
Characteristics and pattern of local relapse
The majority of patients (86%) had a single relapse location within the prostatic fossa while two distinct BTVRELAPSE were identified in the other five patients. Median BTVRELAPSE volume was 0.94 mL (range, 0.1–18.2). The anastomotic site was the most common recurrence site (52.8%), followed by the retro-vesical region (31.7%) and the bladder neck (7%). The median SUV max was 4.9 (range, 2.3–16.1).
shows the 3D mapping of the 41 BTVRELAPSE on a digital reconstructed radiography. The lowest relapse was located 6 mm below the inferior border of the pubic symphysis. On the sagittal plane, 17 relapses were located within the anatomical boundary formed anteriorly by the posterior border of pubic symphysis and the posterior bladder wall, and posteriorly by the anterior border of the mesorectum.
Comparisons of target volumes
As shown in , the volume of the CTVs differed significantly between the three guidelines (p < .0001). The CTVRTOG and CTVFROGG were significantly larger than the CTVEORTC (p < .0001). The volumes suggested by the RTOG and the FROGG did not differ significantly (p = .9).
Distribution of suspected recurrences relative to the contouring guidelines
Eighty-four percent and 83% of BTVRELAPSE were entirely included in the CTVRTOG and CTVFROGG, respectively (p = .5). The CTVEORTC included significantly less BTVRELAPSE than the CTVRTOG (68% versus 84%, p = .006). Three patients had at least one local failure outside the CTVRTOG, six patients outside the CTVEORTC, and three patients outside the CTVFROGG. As shown in table and , the main difference between the different guidelines was due to the variation in coverage of the bladder neck and retrovesical regions. Six out of 10 relapses occurring in the posterior region of the anastomosis were not covered by any of the CTVs ( and ).
Figure 4. Digital reconstructed radiograph showing the spatial distribution of all local relapses with respect to the different CTVs and their respective PTVs: EORTC (top), RTOG (middle), FROGG (bottom).
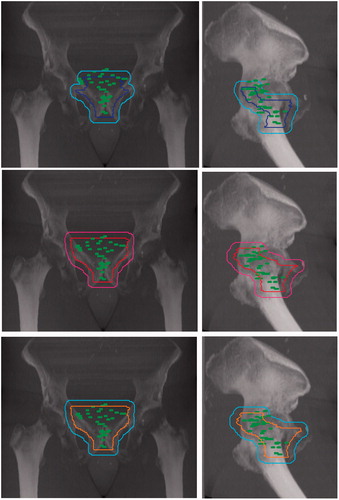
Table 2. Anatomical relapses’ location as seen on 18F-FCH PET/CT.
Table 3. Inclusion of local recurrences by the outlining guidelines with regards to their anatomic site.
Discussion
The main hypothesis of this work was to investigate the sites and spatial distribution of LR after RP, and evaluate how FCH PET/CT in the post-prostatectomy BR setting could influence target volume delineation.
More than half of the suspected relapses occurred at the anastomosis site, and one-third were located in the retro-vesical region in our study. This is in agreement with the report of Wang et al. who found two thirds of relapses around the vesico-urethral anastomosis with the remaining recurrences in the retro-vesical region [Citation13]. Others reported similar results with 66% of PCa recurrences in the anastomotic site, 16% around the bladder neck, and 13% posterior to the trigone [Citation14]. In both of these studies, relapses were confirmed histologically.
Eighty-four percent of relapses seen on 18F-FCH PET were included in the CTV suggested by the RTOG, compared to 83% and 68% in the CTV suggested by the FROGG and the EORTC, respectively. These differences are easily understandable when considering the main variations between the different guidelines. A detailed description of these variations, highlighting their similarities and differences, has been published previously [Citation15] and is summarized in Supplementary Appendix: whereas the RTOG considers the surgical prostatic bed along the posterior aspect of the pubic symphysis to the top of it, the EORTC guidelines include the vesico-urethral anastomosis, the retro-vesical space and the bladder neck, but not the bladder and the supero-anterior area of the prostatic bed. Moreover, the CTVFROGG considers the surgical clips, while the CTVEORTC expansion depends on pathological findings, especially the presence of extra-prostatic extension and/or involved margins. If present, a 5 mm expansion in the positive margin’s direction is recommended. According to our findings, the CTVEORTC is significantly smaller than the CTV recommended by the other two guidelines, due to a less extensive coverage of the prostatic bed in the anterior and posterior directions. Malone et al. also found similar significant variations between the guidelines, resulting in large differences in the doses delivered to OARs with the three different methods. The usual dose constraints to the bladder, as suggested by the RADICALS trial (namely V50 < 80%, V60 < 50%) are indeed more easily met when the EORTC consensus guidelines are used compared to the RTOG recommendations [Citation16].
In one-third of patients without identified macroscopic recurrence on imaging, strictly using the EORTC guidelines to outline the prostatic fossa would certainly allow better sparing of OARs, but could substantially increase the risk for a geographic miss, especially superiorly. In a similar study, Croke et al. showed inadequate coverage of the prostate in 20 evaluated cases of PCa: 41% of the gross tumor volumes (GTVs) visualized on pre-operative MRI were not included in the postoperative CTV outlined following the EORTC guidelines [Citation17]. By comparison, the CTVRTOG provided the best coverage in our study, but still resulted in a geographical miss of around 40% of the suspected recurrences located posteriorly to the anastomosis, and of 26% of the retro-vesical recurrences. To obtain a perfect coverage of all relapses seen in , the CTVRTOG would need to be extended and start 4 cm above the pubic symphysis, extend to 10 mm below the vesico-urethral anastomosis, posteriorly to 1 cm behind the rectal anterior wall, with respect to the anatomic barriers including the periosteum of the pubic symphysis anteriorly and the fascia of the pelvic muscles laterally. Nevertheless, extending the CTV to include all BTVRELAPSE would substantially increase doses to OARs, especially the bladder, and expose patients to a higher risk of urinary and bladder toxicities.
Not all the patients included in this study were treated with SRT at our institution. Therefore, we were not able to perform radiation treatment planning and dose volume histogram analysis to evaluate the BTVRELAPSE dosimetric coverage. As 18F-FCH PET/CT is not performed with full bladder and empty rectum like the planning CT usually is, the enhanced CT of the PET/CT could not be used for the radiation planning either.
18F-FCH PET/CT has been increasingly used during the last decade as a diagnostic tool for early relapse or suspicion of persistent disease after prostatectomy. Nevertheless, because of the physiological biodistribution of the radiopharmaceutical agent, this technique suffers from a modest sensitivity for detecting failure within the prostatic fossa compared to multiparametric MRI. In a recent meta-analysis, Evangelista et al. reported a sensitivity of 75.4% and a specificity of 82% to detect LR [Citation18]. This disappointing accuracy is particularly true in patients with a PSA value of less than 2 ng/mL, or PSA doubling time longer than 6 months, as often observed in patients with local relapse after surgery. False positive rates can even reach 50% to 60% in patients with PSA ≤2 ng/mL and Gleason score ≤7. In our series, median PSA at time of PET/CT was 2.7 ng/mL, and only 11 patients had a PSA less than 2 ng/mL, meaning FCH uptake is highly compatible with true LRs, independently from the Gleason score [Citation10].
In the absence of histologically proven relapse, we concede that our study must be seen as hypothesis generating and we obviously do not recommend a change in target volume definition outside of a clinical trial. Another weakness of this work is the absence of multiparametric MRI data at the time of relapse, as the combination of multiparametric MRI and choline PET/CT is known to enhance the detection of recurrent disease in this setting [Citation19].
The integration of 18F-FCH PET/CT in the RT planning process remains complex, especially regarding the accurate definition of the tumor boundaries. Beyond manual contouring and its highly operator-dependent nature, several semi-automatic methods relying on a SUV fixed [Citation20] or adaptive [Citation21] threshold – based segmentation have been developed in other tumor types. These methods are an attractive option for the radiation oncologist and may not only reduce the operator’s error and subjectivity, but also improve the reproducibility of treatment planning. In a pilot study, Wang et al. showed that the GTVs obtained from several semi-automated segmentation techniques were substantially higher than the manual delineated GTV in partial prostate re-irradiation for local recurrent PCa [Citation22]. Among different automatic segmentation methods available, the gradient-based segmentation technique used in our study is a very promising approach that probably outperforms the signal-to-background ratio (SBR)-based adaptive technique. Indeed, this technique already validated in the field of head neck and lung cancers [Citation12,Citation23] allows for reduced statistical noise and resolution blur compared to fixed or SBR ratio adaptive SUV-based threshold methods.
The development of more specific radiotracers like the 68Gallium labeled prostate-specific membrane antigen (PSMA) usable in PET/CT or the implementation of hybrid PET/MRI could improve the accuracy of recurrence location and its delineation in the near future, even at very low PSA levels below 0.5 ng/mL. This could allow for image-guided integrated dose escalation to the local relapse only.
Conclusions
Inadequate CTV definition is probably a major contributing factor for the substantial local relapse rate after RP. Indeed, none of the three consensus guidelines studied herein ensures a coverage of all suspected local relapses seen on 18F-FCH PET/CT. Considering the EORTC guidelines for delineation of the prostatic bed resulted in a geographic miss in approximately one-third of patients. RTOG- or FROGG consensus outlining should also be applied carefully. Therefore, modifying these consensus guidelines especially in the posterior aspect based on the 18F-FCH PET/CT performed before RT could improve target definition in radiation therapy in the postoperative adjuvant or salvage setting. Performing multiparametric MRI alongside with 18F-FCH PET/CT at the time of BR is also an option to improve the accuracy of outlining.
IONC_A_1385843_Supplementary_Information.docx
Download MS Word (15.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–2041.
- Aus G, Abbou CC, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2005;48:546–551.
- Ploussard G, Agamy MA, Alenda O, et al. Impact of positive surgical margins on prostate-specific antigen failure after radical prostatectomy in adjuvant treatment-naive patients. BJU Int. 2011;107:1748–1754.
- Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76:361–368.
- Poortmans P, Bossi A, Vandeputte K, et al. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol. 2007;84:121–127.
- Miralbell R, Vees H, Lozano J, et al. Endorectal MRI assessment of local relapse after surgery for prostate cancer: a model to define treatment field guidelines for adjuvant radiotherapy in patients at high risk for local failure. Int J Radiat Oncol Biol Phys. 2007;67:356–361.
- Wiltshire KL, Brock KK, Haider MA, et al. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007;69:1090–1099.
- Sidhom MA, Kneebone AB, Lehman M, et al. Post-prostatectomy radiation therapy: consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother Oncol. 2008;88:10–19.
- Mitchell CR, Lowe VJ, Rangel LJ, et al. Operational characteristics of (11)c-choline positron emission tomography/computerized tomography for prostate cancer with biochemical recurrence after initial treatment. J Urol. 2013;189:1308–1313.
- Evangelista L, Cimitan M, Hodolic M, et al. The ability of 18F-choline PET/CT to identify local recurrence of prostate cancer. Abdom Imaging. 2015;40:3230–3237.
- Vali R, Loidl W, Pirich C, et al. Imaging of prostate cancer with PET/CT using (18)F-Fluorocholine. Am J Nucl Med Mol Imaging. 2015;5:96–108.
- Geets X, Lee JA, Bol A, et al. A gradient-based method for segmenting FDG-PET images: methodology and validation. Eur J Nucl Med Mol Imaging. 2007;34:1427–1438.
- Wang J, Kudchadker R, Choi S, et al. Local recurrence map to guide target volume delineation after radical prostatectomy. Pract Radiat Oncol. 2014;4:e239–e246.
- Connolly JA, Shinohara K, Presti JC Jr, et al. Local recurrence after radical prostatectomy: characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology. 1996;47:225–231.
- Croke J, Malone S, Roustan Delatour N, et al. Postoperative radiotherapy in prostate cancer: the case of the missing target. Int J Radiat Oncol Biol Phys. 2012;83:1160–1168.
- Malone S, Croke J, Roustan-Delatour N, et al. Postoperative radiotherapy for prostate cancer: a comparison of four consensus guidelines and dosimetric evaluation of 3D-CRT versus tomotherapy IMRT. Int J Radiat Oncol Biol Phys. 2012;84:725–732.
- Croke J, Maclean J, Nyiri B, et al. Proposal of a post-prostatectomy clinical target volume based on pre-operative MRI: volumetric and dosimetric comparison to the RTOG guidelines. Radiat Oncol. 2014;9:303.
- Evangelista L, Zattoni F, Guttilla A, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013;38:305–314.
- Kanoun SWP, Vrigneaud JM, Depardon E, et al. 18F-Choline PET/CT and multiparametric MRI for the detection of early local recurrence of prostate cancer initially treated by radiotherapy: comparison with systematic 3D-transperineal mapping biopsy. Int J Radiat Oncol Biol Phys. 2017;97:986–994.
- Abgral R, Keromnes N, Robin P, et al. Prognostic value of volumetric parameters measured by 18F-FDG PET/CT in patients with head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2014;41:659–667.
- Daisne JF, Sibomana M, Bol A, et al. Tri-dimensional automatic segmentation of PET volumes based on measured source-to-background ratios: influence of reconstruction algorithms. Radiother Oncol. 2003;69:247–250.
- Wang H, Vees H, Miralbell R, et al. 18F-fluorocholine PET-guided target volume delineation techniques for partial prostate re-irradiation in local recurrent prostate cancer. Radiother Oncol. 2009;93:220–225.
- Wanet M, Lee JA, Weynand B, et al. Gradient-based delineation of the primary GTV on FDG-PET in non-small cell lung cancer: a comparison with threshold-based approaches, CT and surgical specimens. Radiother Oncol. 2011;98:117–125.