Abstract
Background: Proton beam therapy (PBT) reduces normal organ dose compared to intensity modulated radiation therapy (IMXT) for prostate cancer patients who receive pelvic radiation therapy. It is not known whether this dosimetric advantage results in less gastrointestinal (GI) and genitourinary (GU) toxicity than would be expected from IMXT.
Material and methods: We evaluated treatment parameters and toxicity outcomes for non-metastatic prostate cancer patients who received pelvic radiation therapy and enrolled on the PCG REG001-09 trial. Patients who received X-ray therapy and/or brachytherapy were excluded. Of 3210 total enrolled prostate cancer patients, 85 received prostate and pelvic radiation therapy exclusively with PBT. Most had clinically and radiographically negative lymph nodes although 6 had pelvic nodal disease and one also had para-aortic involvement. Pelvic radiation therapy was delivered using either 2 fields (opposed laterals) or 3 fields (opposed laterals and a posterior beam). Median pelvic dose was 46.9 GyE (range 39.7–56) in 25 fractions (range 24–30). Median boost dose to the prostate +/− seminal vesicles was 30 GyE (range 20–41.4) in 16 fractions (range 10–24).
Results: Median follow-up was 14.5 months (range 2.8–49.2). Acute grade 1, 2, and 3 GI toxicity rates were 16.4, 2.4, 0%, respectively. Acute grade 1, 2, and 3 GU toxicity rates were 60, 34.1, 0%, respectively.
Conclusions: Prostate cancer patients who receive pelvic radiation therapy using PBT experience significantly less acute GI toxicity than is expected using IMXT. Further investigation is warranted to confirm whether this favorable acute GI toxicity profile is related to small bowel sparing from PBT.
Introduction
Whole pelvic radiation therapy (WPRT) is commonly used for intermediate and high-risk prostate cancer patients who have an increased risk of subclinical pelvic nodal involvement despite several randomized trials not showing a survival benefit compared to prostate only radiation therapy (PORT) [Citation1–3]. The inclusion of pelvic nodes exposes a larger volume of the pelvis to unintentional and unwanted radiation dose, and this has been associated with increased gastrointestinal (GI) and genitourinary (GU) toxicities with conventional X-ray therapy [Citation4]. Intensity modulated radiation therapy (IMXT) has improved pelvic organ sparing compared to older X-ray therapy techniques, especially that of the small bowel [Citation5,Citation6] and this has consequently reduced morbidity [Citation7–13]. However, the price paid for the high-dose conformality of IMXT is that low doses up to around 20 Gy are spread to a large volume of the normal pelvis. While the significance of this ‘low dose bath’ may be overlooked, a strong relationship between low dose received by small bowel and acute GI toxicity has been clearly established and therefore should be a high priority during treatment planning [Citation14–18]. With this in mind it should not be surprising that acute GI toxicity is consistently reported as being higher in prostate cancer patients who receive WPRT compared to those who receive PORT. For instance, investigators at the University of Pennsylvania who treated patients with IMXT reported a 50% incidence of acute grade 2 GI toxicity among WPRT patients compared to 13% among PORT patients (p = .006) [Citation11].
Proton beam therapy (PBT) has the distinct benefit over X-ray therapy of having no exit dose distal to the target. Published results of PBT studies for prostate cancer patients have been favorable and indicate that patients maintain excellent quality of life after treatment [Citation19–24]. Investigators at M.D. Anderson Cancer Center, for instance, published quality of life and toxicity outcomes from 226 men who received PBT of which only one experienced grade 3 GI toxicity and none experienced grade 3 GU toxicity [Citation21]. Long-term outcomes of image-guided accelerated hypofractionated PBT in 215 low- and intermediate-risk prostate cancer patients were recently reported from the University of Florida that included 5-year freedom from 95.9% biochemical disease progression, 0.5% grade 3 or higher GI toxicity, and 1.7% grade 3 or higher GU toxicity [Citation24].
Target volumes from contemporary prostate cancer PBT studies, however, have predominantly included the only prostate with or without a portion of the seminal vesicles. As such, there is a lack of published cancer-related and toxicity outcomes for prostate cancer patients who receive pelvic nodal irradiation. Based on published dosimetric comparisons showing that pelvic radiation delivered with PBT has the ability to provide remarkable small bowel sparing compared to IMXT for prostate cancer patients [Citation25–28], our hypothesis for this study was that acute GI toxicity is less for patients treated with PBT compared to historical IMRT control.
Material and methods
A multi-institutional prospective database of patients treated with PBT on a registry trial was queried to identify patients who received definitive radiation therapy for prostate cancer. Patients were required to have received pelvic lymph node irradiation for inclusion in this study. Patients who received X-ray therapy or brachytherapy were excluded to create a study population that was exclusively treated with PBT. Patients who received pelvic re-irradiation were also excluded.
Of a total 3210 prostate cancer patients in the registry trial database, 85 met criteria for inclusion. These 85 patients were treated across 7 institutions from 2010 to 2016; the median number treated per institution was 12 (range, 2–23). The number of patients included in this study treated by year increased from 6 (2010–2012) to 27 (2013–2014) to 42 (2015–2016).
Patient and tumor characteristics in addition to treatment details were collected (). Initial staging included computerized tomography (CT) scan in 72.9% of patients and magnetic resonance imaging (MRI) scan in 58.8%. CT simulation was performed in the supine position with a custom immobilization device. Patients were advised to have an empty rectum prior to simulation and treatment. Instructions for bladder filling varied between institutions, but generally consisted of the patient either having a comfortably full bladder, or otherwise voiding and then drinking approximately 470 cm3 (16 ounces) of water prior to simulation and treatment.
Table 1. Patient, tumor, and treatment characteristics.
The clinical target volume (CTV) encompassing elective pelvic lymph nodes was contoured as per Radiation Therapy Oncology Group (RTOG) guidelines with the superior border at the L5/S1 interface [Citation29]. The only exception to this was 1 institution that instead used the caudal extent of the sacroiliac joints; this institution contributed 11 patients. A 5 mm planning target volume (PTV) expansion was added to the elective pelvic lymph node CTV. A boost was delivered to the entire prostate and typically at least the proximal 2 cm of the seminal vesicles (SVs) although the extent of SV coverage varied and included the entire SV in some patients depending on the extent of disease including direct SV invasion. The PTV expansion of the prostate ± SV was 5–6 mm except 3–5 mm posteriorly. For the node-positive patients, all grossly involved lymph nodes were contoured and a 5–7 mm PTV expansion was used.
Pelvic radiation therapy was delivered using either 2 fields (opposed laterals) or 3 fields (opposed laterals and a posterior beam). The prostate ± SV and gross nodal boosts were typically delivered using lateral fields. Beam-specific PTV expansions consisting of 3% Hounsfield unit density uncertainty plus 1 mm were used to account for distal and proximal range uncertainty.
The registry trial did not require documentation of intraprostatic fiducial markers, rectal balloon, or hydrogel rectal spacer utilization. However, most treating institutions routinely recommended that fiducial markers be placed to facilitate daily image guidance with orthogonal kilovoltage X-rays or cone beam CT (available at 2 institutions starting in 2015). Patient setup was most commonly done first with alignment to bony and then to fiducial markers if present. Based on institutional practice patterns and the number of patients contributed by institution, we estimate that approximately 20% of the patients were treated with a hydrogel rectal spacer and 20% were treated with a rectal balloon; no institution used both in the same patient.
Acute GI and GU toxicities were determined for each patient according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 criteria () and were considered to have occurred during or within 3 months from the completion of PBT. The maximum toxicity reported for each patient was evaluated. A physician prospectively scored all toxicities at the time of initial consultation, at least once weekly during treatment, and at each follow-up encounter. Follow-up was performed typically every 3 months and included at least yearly digital rectal examination and PSA evaluation at least every 6 months. Importantly, toxicity evaluation performed outside of the treating institution at any time was not considered for this analysis.
Table 2. The most severe acute and late gastrointestinal (GI) and genitourinary (GU) toxicity among prostate cancer patients receiving pelvic nodal irradiation with proton beam therapy.
While there was variability in dose-volume constraints across institutions, the most commonly used were: bladder V80 < 8cc, V70 < 10%, V50 < 35%; rectum V70 < 10%, V50 < 35%; femoral heads V50 < 1 cc; penile bulb mean 52.5 Gy; small bowel maximum 55 Gy.
Results
Patient and tumor characteristics are summarized in . Briefly, the median age was 69 years (range, 53.9–79.9) and nearly 80% had a Gleason score of ≥8 while almost two-thirds had clinical T2-3 disease. The median prostate specific antigen (PSA) immediately prior to PBT was 8.21 ng/mL (range, 0.1–126.18) although 51 patients (60%) received neoadjuvant ADT. Six patients (7.1%) were staged as N1 although details about the extent of nodal disease including size, number, and location in the pelvis were not available; para-aortic nodal involvement was present in one patient with pelvic nodal disease.
Most patients were treated with pencil beam scanning (PBS) (68.2%); the remainder was treated with uniform scanning (US) (31.8%). Only one institution exclusively treated patients with US while two that initially used US transitioned to only using PBS around 2014–2015. The four other institutions treated patients only with PBS.
The median prescribed dose to pelvis was 46.9 GyE (range, 39.7–56) in 25 fractions (range, 24–30) of 1.8 Gy each (range, 1.3–3.2). The median boost dose to the prostate was 30 GyE (range, 20–41.4) in 16 fractions (range, 10–24) of 1.8 Gy each (range, 1.6–2.0). The median total dose to the prostate was 79.4 GyE (range, 70–80.2) in 44 fractions (range, 35–54). Each of the 6 node-positive patients received a boost to the clinically involved lymph node(s) to a total median dose of 66.5 GyE (range, 59.4–70.6) in 37 fractions (range, 30–44); 4 of the 6 were treated with US.
The median follow-up for all patients was 14.5 months (range 2.8–49.2). The numbers of patients with follow-up of at least 12 and 24 months were 43 (50.6%) and 18 (21.2%), respectively.
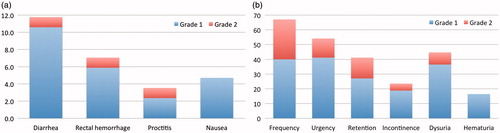
The maximum GI and GU toxicities for each patient are listed in . Acute grade 2 GI toxicity was reported in only 2 patients (2.4%) who experienced diarrhea or proctitis, respectively. There was no acute grade 3 or higher GI toxicity. Acute grade 2 GU toxicity occurred in 29 patients (34.1%), and most commonly consisted of urinary frequency. There was no acute grade 3 or higher GU toxicity. Among these 29 patients the median baseline International Prostate Symptom Score (IPSS) immediately prior to PBT was 8 (range, 0–25), which was significantly higher than all other patients (8 vs. 4; p = .01). Of note, 5 of the 6 patients with node-positive disease experienced acute grade 2 GU toxicity despite their low median baseline IPSS of 3.5 (range, 0–23). The frequency of all individual GI and GU toxicities (not limited to the maximum) in each patient are shown in and , respectively.
Discussion
Because of an increased risk of pelvic nodal involvement in patients with intermediate and high-risk prostate cancer, pelvic lymph nodal irradiation remains clinically intriguing even though several randomized trials have failed to demonstrate that a survival benefit exists for whole pelvic radiation therapy (WPRT) compared to prostate only radiation therapy (PORT) [Citation1–3]. If it were not for the fear of increasing the complication rate with pelvic nodal irradiation, this would be less of an issue.
The tradeoff for treating pelvic lymph nodes vs. only the prostate is a higher dose to pelvic organs, especially the small bowel and bladder, which consequently increases the likelihood of gastrointestinal (GI) and genitourinary (GU) morbidity. In Radiation Therapy Oncology Group (RTOG) 9413 that used a conventional X-ray technique, the incidence of acute grade 2 GI toxicity in the WPRT arm was 16.3% higher than in the PORT arm and there was also a trend towards increased late grade 3 GU toxicity [Citation30]. Some studies have reported no difference in toxicity between WPRT and PORT although this might be explained by treatment technique [Citation31,Citation32]. For example, the GETUG-01 study reported no difference in toxicity with the superior border of the pelvic field being placed at the S1/S2 interface instead of the more commonly used L5/S1 interface, thus including less normal tissue in the pelvic field [Citation32].
Outcomes of PBT for localized prostate cancer have been at least comparable to that of IMXT, although most published reports have focused on patients who received treatment to the prostate ± seminal vesicles and excluding pelvic lymph nodes. The most mature data of PBT for prostate cancer comes from Loma Linda although most were treated with combination of X-rays and protons (n = 731). With median follow-up of 63 months the biochemical failure free survival was 75% while grade 3 toxicity was minimal [Citation19]. Favorable quality of life and toxicity outcomes have more recently been reported by several groups [Citation21,Citation24,Citation33–35]. Investigators at the University of Florida found minimal changes in bowel, urinary, and sexual quality of life scores after PBT to the prostate [Citation33] and a later analysis from the same institution reported low rates of grade 2 or higher GI toxicity that predominantly consisted of transient rectal bleeding [Citation34]. Gray and colleagues observed a clinically meaningful reduction in bowel quality of life at initial follow-up in patients who received X-ray therapy although there was no such decrease in patients who received PBT [Citation35]. However, robust toxicity and quality of life data do not exist specifically for prostate cancer patients who receive pelvic nodal irradiation.
To our knowledge this is the first prospective assessment of toxicity in prostate cancer patients who received dose-escalated PBT with inclusion of pelvic lymph nodes. Only two patients (2.4%) experienced grade 2 or higher acute GI toxicity, which is striking compared to rates as high as 50% from IMXT () [Citation8,Citation11,Citation36]. As a matter of fact, the incidence of acute grade 2 or higher GI toxicity is consistent with that reported after treating the prostate alone [Citation24,Citation37]. Although we did not distinguish rectal from non-rectal toxicities in our analysis, others have reported low rates of rectal toxicity as a result of PBT for prostate cancer [Citation34]. The very low incidence of GI toxicity is even more intriguing when one considers that (1) proton target volumes are typically larger than their X-ray counterparts due to additional beam-specific uncertainty margin, and (2) that several patients had clinically positive pelvic lymph nodes which were boosted to a median dose exceeding 66 GyE, strongly suggesting that PBT was able to spare a large volume of normal pelvic tissue, especially small bowel. We recognize that these outcomes are difficult to interpret in the context of our study with the lack of dosimetric information.
Table 3. Acute gastrointestinal (GI) and genitourinary (GU) toxicity in prostate cancer patients treated to pelvic lymph nodes with intensity modulated radiation therapy compared to the current study.
While the acute GI toxicity rate reported in our study is low, this is not unexpected given then expected sparing of small bowel achieved with PBT. A strong relationship between low small bowel dose and acute GI toxicity has been well established in rectal [Citation14,Citation15], anal canal [Citation16], and gynecologic [Citation17] cancer patients. Moreover, Fiorino et al. found that the V40-50 of the intestinal cavity was a main predictor of acute bowel toxicity in a study of 191 prostate cancer patients who received WPRT [Citation38]. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) guidelines recommend restricting the volume of small bowel receiving at least 15 Gy; however, this is routinely not achievable in pelvic patients even using the most sophisticated X-ray techniques [Citation39].
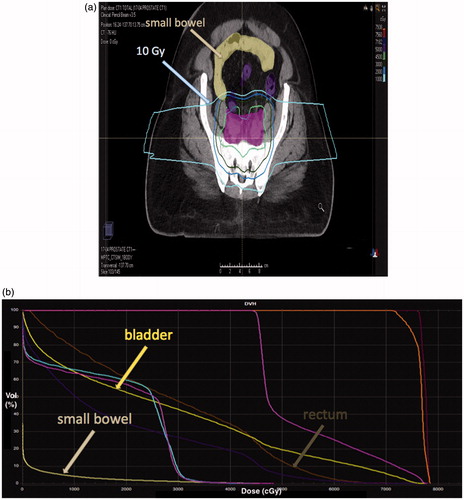
In silico comparisons of PBT and IMXT for prostate and elective pelvic lymph node irradiation have demonstrated that small bowel sparing at low and moderate doses is superior with PBT. Chera and colleagues reported that IMXT plans delivered a significantly higher small bowel V10 (242.29 vs. 85.61 cc; p = .006), V20 (191.86 vs. 58.93 cc; p = .0027), and V30 (123.23 vs. 42.72 cc; p = .0186) compared to passively scattered PBT [Citation26]. Subsequent publications by Vees et al. [Citation27] and Widesott et al. [Citation28] support these findings. shows a patient in which a significant volume of small bowel was spared from the 10 Gy isodose line; this is likely representative of what was achieved in the remainder of patients in this study.
Because urethral dose is a primary driver of GU toxicity, it is not surprising that acute GU toxicity observed in our study (34.1%) was consistent with what has been reported by IMXT series (13–50%) [Citation8–12]. Zelefsky et al. reported no reduction in acute GU toxicity using IMXT compared to 3DCRT despite improved bladder dosimetry [Citation40]. Reported GU toxicity with image-guided PBT for prostate cancer has also been similarly low [Citation41,Citation42]. Furthermore, although PBT reduces the volume of bladder exposed to low doses compared to photon therapy [Citation26–28] a clear relationship between GU toxicity and low bladder dose has not been demonstrated [Citation11,Citation43]. We note that the patients who experienced more significant GU morbidity in our study had higher baseline IPSS values. Also, most of the node-positive patients who received a nodal boost experienced grade 2 GU toxicity.
While PBT almost certainly played a pivotal role in minimizing toxicities, other treatment-related factors should also be considered. First, toxicity may have been reduced on account of hydrogel rectal spacer use [Citation44]. Second, tight target volume margins were used, which was made feasible by the regular use of fiducial markers and daily image guidance including cone beam CT at some centers [Citation36]. Third, more than two-thirds of the patients were treated with PBS although whether toxicities would have differed if only PBS was used, especially in the context of treating node-positive patients, is unknown. Finally, it is possible that ADT may have affected our toxicity outcomes, especially in view of the fact that nearly 80% of the patients in our study received concurrent ADT. A secondary analysis of 3 RTOG studies (8531, 8610, 9202) showed that patients who received short-term ADT were less likely to experience grade 3 or higher GI (p = .0006) and GU (p = .0037) toxicity compared to those who received RT alone [Citation45]. On the contrary, Feigenberg and colleagues reported increased 5-year risk of grade 2 or higher GI (26 vs. 17%; p = .017) and GU (14 vs. 8%; p = .02) morbidity with the use of long-term ADT [Citation46].
There are limitations of this study, the foremost of which is the absence of dosimetric information, especially with regards to small bowel dose. However, all treating institutions used similar treatment planning goals. Second, although toxicity scoring was done in a prospective manner because of the multi-institutional nature of the registry trial the scoring was done by many different people, which likely influenced the overall scoring. Third, our follow-up was rather short (median 14.5 months) and therefore, we cannot adequately assess late toxicities at this time, although certainly intend to in the future.
In conclusion, pelvic lymph node PBT results in dramatically reduced acute GI toxicity as compared to what is expected when similar patients are treated with IMXT for prostate cancer. These data provide support for prospective evaluation of outcomes in prostate cancer patients treated with PBT to the pelvic lymph nodes. An ongoing trial (NCT02874014) is evaluating PBT for treatment of the prostate and pelvic nodes with the primary endpoint of late grade 3 or higher GI and GU toxicity.
Disclosure statement
The authors report no conflict of interest.
References
- Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Update of the long-term survival results of the GETUG-01 randomized study. Int J Radiat Oncol Biol Phys. 2016;96:759–769.
- Lawton CA, DeSilvio M, Roach M, 3rd, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655.
- Asbell SO, Martz KL, Shin KH, et al. Impact of surgical staging in evaluating the radiotherapeutic outcome in RTOG #77-06, a phase III study for T1BN0M0 (A2) and T2N0M0 (B) prostate carcinoma. Int J Radiat Oncol Biol Phys. 1998;40:769–782.
- Aizer AA, Yu JB, McKeon AM, et al. Whole pelvic radiotherapy versus prostate only radiotherapy in the management of locally advanced or aggressive prostate adenocarcinoma. Int J Radiat Oncol Biol Phys. 2009;75:1344–1349.
- Ashman JB, Zelefsky MJ, Hunt MS, et al. Whole pelvic radiotherapy for prostate cancer using 3D conformal and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:765–771.
- Nutting CM, Convery DJ, Cosgrove VP, et al. Reduction of small and large bowel irradiation using an optimized intensity-modulated pelvic radiotherapy technique in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48:649–656.
- Muren LP, Wasbo E, Helle SI, et al. Intensity-modulated radiotherapy of pelvic lymph nodes in locally advanced prostate cancer: planning procedures and early experiences. Int J Radiat Oncol Biol Phys. 2008;71:1034–1041.
- Sanguineti G, Endres EJ, Parker BC, et al. Acute toxicity of whole-pelvis IMRT in 87 patients with localized prostate cancer. Acta Oncol. 2008;47:301–310.
- Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:57–64.
- Bayley A, Rosewall T, Craig T, et al. Clinical application of high-dose, image-guided intensity-modulated radiotherapy in high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:477–483.
- Deville C, Both S, Hwang WT, et al. Clinical toxicities and dosimetric parameters after whole-pelvis versus prostate-only intensity-modulated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:763–772.
- Ishii K, Ogino R, Hosokawa Y, et al. Comparison of dosimetric parameters and acute toxicity after whole-pelvic vs prostate-only volumetric-modulated arc therapy with daily image guidance for prostate cancer. Br J Radiol. 2016;89:20150930.
- Wang-Chesebro A, Xia P, Coleman J, et al. Intensity-modulated radiotherapy improves lymph node coverage and dose to critical structures compared with three-dimensional conformal radiation therapy in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:654–662.
- Robertson JM, Lockman D, Yan D, et al. The dose-volume relationship of small bowel irradiation and acute grade 3 diarrhea during chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:413–418.
- Baglan KL, Frazier RC, Yan D, et al. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:176–183.
- Olsen JR, Moughan J, Myerson RJ, et al. Predictors of radiotherapy-related gastrointestinal toxicity from anal cancer DP-IMRT: secondary analysis of RTOG 0529. J Clin Oncol. 2014;32.
- Roeske JC, Bonta D, Mell LK, et al. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol. 2003;69:201–207.
- Tho LM, Glegg M, Paterson J, et al. Acute small bowel toxicity and preoperative chemoradiotherapy for rectal cancer: investigating dose-volume relationships and role for inverse planning. Int J Radiat Oncol Biol Phys. 2006;66:505–513.
- Slater JD, Rossi CJ, Jr., Yonemoto LT, et al. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59:348–352.
- Mendenhall NP, Hoppe BS, Nichols RC, et al. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88:596–602.
- Pugh TJ, Munsell MF, Choi S, et al. Quality of life and toxicity from passively scattered and spot-scanning proton beam therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;87:946–953.
- Goenka A, Newman NB, Fontanilla H, et al. Patient-reported quality of life after proton beam therapy for prostate cancer: the effect of prostate size. Clin Genitourinary Cancer. 2017.
- Vargas CE, Hartsell WF, Dunn M, et al. Image-guided hypofractionated proton beam therapy for low-risk prostate cancer: analysis of quality of life and toxicity, PCG GU 002. Rep Pract Oncol Radiother. 2016;21:207–212.
- Henderson RH, Bryant C, Hoppe BS, et al. Five-year outcomes from a prospective trial of image-guided accelerated hypofractionated proton therapy for prostate cancer. Acta Oncol. 2017;56:963–970.
- Hashimoto S, Shibamoto Y, Iwata H, et al. Whole-pelvic radiotherapy with spot-scanning proton beams for uterine cervical cancer: a planning study. J Radiat Res. 2016;57:524–532.
- Chera BS, Vargas C, Morris CG, et al. Dosimetric study of pelvic proton radiotherapy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:994–1002.
- Vees H, Dipasquale G, Nouet P, et al. Pelvic lymph node irradiation including pararectal sentinel nodes for prostate cancer patients: treatment optimization comparing intensity modulated X-rays, volumetric modulated arc therapy, and intensity modulated proton therapy. Technol Cancer Res Treat. 2015;14:181–189.
- Widesott L, Pierelli A, Fiorino C, et al. Helical tomotherapy vs. intensity-modulated proton therapy for whole pelvis irradiation in high-risk prostate cancer patients: dosimetric, normal tissue complication probability, and generalized equivalent uniform dose analysis. Int J Radiat Oncol Biol Phys. 2011;80:1589–1600.
- Lawton CA, Michalski J, El-Naqa I, et al. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–387.
- Roach M, 3rd, DeSilvio M, Lawton C, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21:1904–1911.
- Mantini G, Tagliaferri L, Mattiucci GC, et al. Effect of whole pelvic radiotherapy for patients with locally advanced prostate cancer treated with radiotherapy and long-term androgen deprivation therapy. Int J Radiat Oncol Biol Phys. 2011;81:e721–e726.
- Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25:5366–5373.
- Hoppe BS, Nichols RC, Henderson RH, et al. Erectile function, incontinence, and other quality of life outcomes following proton therapy for prostate cancer in men 60 years old and younger. Cancer. 2012;118:4619–4626.
- Colaco RJ, Hoppe BS, Flampouri S, et al. Rectal toxicity after proton therapy for prostate cancer: an analysis of outcomes of prospective studies conducted at the university of Florida Proton Therapy Institute. Int J Radiat Oncol Biol Phys. 2015;91:172–181.
- Gray PJ, Paly JJ, Yeap BY, et al. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer. 2013;119:1729–1735.
- Chung HT, Xia P, Chan LW, et al. Does image-guided radiotherapy improve toxicity profile in whole pelvic-treated high-risk prostate cancer? Comparison between IG-IMRT and IMRT. Int J Radiat Oncol Biol Phys. 2009;73:53–60.
- Coen JJ, Bae K, Zietman AL, et al. Acute and late toxicity after dose escalation to 82 GyE using conformal proton radiation for localized prostate cancer: initial report of American College of Radiology Phase II study 03-12. Int J Radiat Oncol Biol Phys. 2011;81:1005–1009.
- Fiorino C, Alongi F, Perna L, et al. Dose-volume relationships for acute bowel toxicity in patients treated with pelvic nodal irradiation for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:29–35.
- Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76:S101–S107.
- Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116.
- Henderson RH, Hoppe BS, Marcus RB, Jr, et al. Urinary functional outcomes and toxicity five years after proton therapy for low- and intermediate-risk prostate cancer: results of two prospective trials. Acta Oncol. 2013;52:463–469.
- Bryant C, Smith TL, Henderson RH, et al. Five-year biochemical results, toxicity, and patient-reported quality of life after delivery of dose-escalated image guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2016;95:422–434.
- Guckenberger M, Baier K, Richter A, et al. Does intensity modulated radiation therapy (IMRT) prevent additional toxicity of treating the pelvic lymph nodes compared to treatment of the prostate only? Radiat Oncol. 2008;3:3.
- Chung H, Polf J, Badiyan S, et al. Rectal dose to prostate cancer patients treated with proton therapy with or without rectal spacer. J Appl Clin Med Phys. 2017;18:32–39.
- Lawton CA, Bae K, Pilepich M, et al. Long-term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: analysis of radiation therapy oncology group studies 85-31, 86-10, and 92-02. Int J Radiat Oncol Biol Phys. 2008;70:437–441.
- Feigenberg SJ, Hanlon AL, Horwitz EM, et al. Long-term androgen deprivation increases Grade 2 and higher late morbidity in prostate cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:397–405.