Abstract
Background: Adaptive (ART) and image guided radiation therapy (IGRT) can improve target coverage and reduce unnecessary irradiation of organs at risk (OAR). The purpose of this study is to report the results of using mid-course imaging and a novel workflow with cone-beam computed tomography (CBCT) surveillance of dose to OAR to trigger adaptive replanning in head and neck radiotherapy.
Material and methods: Impact of radiation therapist (RTT) managed match protocol and mid-course imaging was assessed in two cohorts of consecutive patients receiving RT to the head and neck region, using computed tomography (CT) and CBCT-based dose verification respectively. In the CBCT cohort, patients at high risk of xerostomia received weekly dose surveillance, while low-risk patients received a mid-course CBCT review. For weekly surveillance, predicted total doses to parotid glands, spinal cord and brainstem were calculated. If predicted mean dose to parotid glands increased by >2 Gy or constraints to brainstem or spinal cord were exceeded, replanning was performed.
Results: None had replanning triggered by mid-course imaging. In the CBCT cohort, weekly surveillance of 40 patients yielded minimal reduction in mean dose to parotid glands of 0.65 Gy (range0.4–1 Gy ) for three patients. Patients were surveilled averagely 4.5 times during treatment. Time consumption per CBCT/week was 22 min (range 17–38). Number of patients needed to see to achieve any dose reduction to parotid glands was 13 or the equivalent of 22 working-hours.
Conclusion: The tested dose surveillance algorithm resulted in a minimal dose reduction ( ≤1 Gy) to parotid glands for three of 40 patients. The proposed algorithm and workflow is thus not sustainable. Mid-course dose verification did not provide added benefit and can be safely omitted in the presence of closely monitored daily IGRT. Daily image guidance and match protocol is a safe and efficient method for identifying patients requiring adaptive replanning.
Background
Radiation therapy (RT) is the primary treatment for most patients with head and neck squamous cell carcinoma (HNSCC), providing a high degree of loco-regional control either in combination with chemotherapy or following surgery. However, due to irradiation of adjacent normal tissues including parotid glands and swallowing muscles, side effects such as xerostomia and dysphagia remain relatively common, severely impacting quality of life for long-term survivors [Citation1–4]. Improvements in treatment delivery with intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) allow for improved sparing of normal tissues without compromising target coverage due to steep dose gradients [Citation5]. These techniques, however, do not account for the substantial anatomical changes, which can occur during the course of RT, potentially causing underdosage of target volumes and over dosage of normal tissues [Citation6–8].
Image guided (IGRT) and adaptive radiation therapy (ART) are strategies used for limiting these problems. IGRT reduces the risk of a geographical miss and has the potential to reduce margins [Citation9]. Although demanding in terms of physician and physicist workload, ART with even a single mid-course computed tomography (CT) scan can improve target coverage and organ at risk sparing [Citation10–12]. Despite considerable efforts, no consensus has been reached on how to best implement ART in the head and neck region regarding patient selection, optimal timing and frequency of adaptive replanning [Citation13].
Today, state of the art IGRT includes daily kilovoltage cone-beam CT (CBCT), allowing for accurate treatment setup and better visualization of soft tissues, compared to earlier imaging techniques or less frequent imaging [Citation14,Citation15]. IGRT can be supplemented with mid-course CT imaging for verification of dose to target volumes and critical organs at risk (OAR). CBCT imaging can be optimized, allowing for both delineation and deformable image registration (DIR) assisted propagation of soft tissue organ contours, as well as dose recalculation in the head and neck region with an accuracy of 2–3% for clinically relevant dose-volume parameters compared to CT-based dose planning [Citation16–19]. This enables more convenience in adaptive workflows, as it could reduce the need for mid-course CTs and allow for a more thorough monitoring of parotid gland dose changes.
The purpose of this study was to describe and quantify the effects of an RTT managed match protocol and mid-course imaging on the number of adaptive replannings performed in an IGRT workflow. Furthermore, to determine the efficiency of a novel parotid gland dose surveillance algorithm as an additional trigger for adaptive replanning in a subgroup of patients.
Material and methods
Patients
The study includes two patient cohorts, who were both treated with daily CBCT-based IGRT, while the method for offline dose verification differed between the two. One cohort (CT cohort) had CT-based dose verification with a mid-course CT scan while the other (CBCT cohort) had CBCT-based dose verification, either mid-course or weekly, depending on a priori xerostomia risk. Both cohorts were used to study the impact of RTT managed match protocol and mid-course imaging, while the novel dose surveillance algorithm was studied in the CBCT cohort’s weekly surveillance group exclusively.
The CT cohort included all 93 patients treated with curatively intended RT for malignant tumors in the head and neck region during the first six months of 2016. Patient data were prospectively gathered.
For the CBCT cohort, the study included 60 consecutive patients receiving curatively intended RT for malignant tumors in the head and neck region from December 3 2016 to March 28 2017. Forty-six had radiation as part of their primary treatment for HNSCC, two had recurrent HNSCC and the remaining 12 had non-HNSCC cancers, mostly squamous cell skin cancers. All patients were staged using the seventh edition of the Union for International Cancer Control’s staging manual.
Patient characteristics are shown in .
Table 1. Patient characteristics, n (%).
Planning and treatment
For planning, all patients received an intravenous contrast-enhanced CT (3 mm slice thickness) from the vertex to the carina while fixed in the treatment position with thermoplastic mask. Magnetic resonance and positron emission tomography scans were available if deemed necessary for RT planning. Gross tumor volume (GTV) was defined as all macroscopic tumor on imaging and clinical examination. For HNSCC patients, high-risk clinical target volume (CTV1) was defined as the GTV plus a 5 mm concentric margin expansion, excluding natural barriers like air cavities and uninvolved bone. Intermediate risk CTV (CTV2) was defined as CTV1 plus an additional 5 mm margin expansion, excluding natural barriers. Elective CTV (CTV3) was defined according to consensus guidelines [Citation20]. In general, planning target volumes (PTV) were generated for each CTV by adding 5 mm concentric margin expansions, with a smaller 3 mm margin expansion being used in case of target volumes in close proximity to eyes, chiasm or brainstem. All treatment planning was performed in Eclipse (v. 13, Varian Medical Systems, Palo Alto, CA, USA).
All patients underwent VMAT or IMRT. HNSCC patients receiving primary RT had either accelerated RT with 66/68 Gy in 33/34 fractions, six fractions weekly or accelerated hyper fractionated RT with 76 Gy in 56 fractions, 10 fractions weekly. Medical treatment with the hypoxic sensitizer Nimorazole and/or weekly Cisplatin was prescribed as appropriate in concordance with the guidelines of the Danish Head and Neck Cancer Group, DAHANCA (https://www.dahanca.oncology.dk). Postoperative HNSCC patients and non-HNSCC patients were treated with conventionally fractionated RT, 60–66 Gy in 30–33 fractions, five fractions weekly, without medical treatment.
Normal tissue dose constraints followed DAHANCA guidelines. Specifically, dose constraints to the parotid glands were Dmean ≤26 and 20 Gy for both parotid glands and contralateral parotid gland, respectively [Citation21].
Daily IGRT and match protocol
All patients had daily treatment setup guided by CBCT as a part of a match protocol performed by the treating RTTs per in-house guidelines. Irregularities such as needing to manually adjust treatment position after bony anatomy match, couch shifts >3° or >1 cm change in body outline were recorded. Patient specific tolerances of 3–5 mm on bony anatomy in proximity to target volumes were applied. In the event of systematic irregularities on three consecutive treatment fractions a physicist would be called to review dosimetric consequences and order a renewed CT scan if deemed necessary, triggering adaptive replanning.
CT cohort dose verification
If no irregularities occurred, patients in the CT cohort underwent a mid-course CT scan for verification of the original dose plan, triggering replanning if potential and clinically relevant underdose to target volumes or overdose to spinal cord or brainstem were found.
CBCT cohort dose verification
Patients in the CBCT cohort underwent weekly surveillance of dose to target volumes, brainstem, spinal cord and parotid glands if mean dose to at least one parotid gland was between 10–40 Gy. If this criterion was not met (i.e., very high or very low mean doses to both parotid glands), limited change to parotid gland normal tissue complication probability (NTCP) would be expected as a result of adaptive replanning, since minimal reduction of function occurs at 10 Gy, gradually worsening to a strong reduction at doses >40 Gy [Citation21]. These patients would be assigned to a single mid-course CBCT review of dose to target volumes, brainstem and spinal cord. For weekly dose surveillance, the original treatment plan was recalculated for a CBCT of the week in eclipse with dedicated CBCT calibration and the plan was transferred according to the online registrations performed at treatment. Images and dose were transferred to the image registration software MIM (MIM v. 6.4.4, MIM software inc., Cleveland, OH, USA) for propagation of contours from the planning CT to the relevant CBCT, followed by manual correction where needed and extraction of DVH parameters, including mean and max doses. Under the assumption that this dose of the week would remain constant for the remaining treatment fractions and taking into account the already delivered dose from previous weeks, predicted total dose was calculated. In the event of >2 Gy predicted mean dose increase to parotid glands (corresponding to up to 8% NTCP increase [Citation22,Citation23]) or predicted max dose increase to the brainstem or spinal cord, adaptive replanning would be triggered. In case of limited field of view or poor image quality, a mid-course CT scan was performed for dose verification. For each weekly dose surveillance session, time consumption was measured from selection of CBCT of the week until and including calculation of predicted dose.
Results
CT cohort
To study the effects of mid-course CT and match protocol, the CT cohort of 93 patients was reviewed. Sixteen of the 93 patients (17%) were replanned once, 12 due to systematic irregularities identified by the match protocol and four due to issues related to the thermoplastic mask and fixation. None were replanned due to the mid-course CT dose verification alone.
CBCT cohort
In the CBCT cohort, 40 patients were assigned to weekly dose surveillance and 20 to the single mid-course CBCT surveillance [].
Figure 1. Grouping and outcome of the CBCT cohort. IGRT: image guided radiation therapy. *Two patients had two replans due to match protocol. †Two patients had two replans due to match protocol and fixation issues.
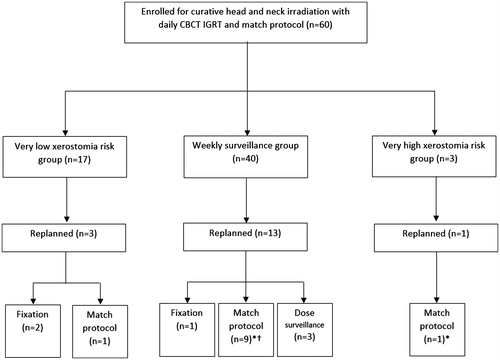
In the CBCT cohort, a total of 21 replans were performed in 17 of the 60 patients (28%), four had two replans during the course of treatment. Thirteen were triggered due to match protocol irregularities and five were caused by various mask and fixation issues. Three patients, all p16 positive stage IVA HNSCC (two oropharyngeal and one hypopharyngeal), were replanned once due to increasing parotid gland mean dose identified during weekly dose surveillance. shows the distribution of replannings over time after start of RT.
Table 2. Timing and reasons for adaptive replanning in the CBCT cohort. CBCT: cone-beam computed tomography.
For the three patients identified during weekly dose surveillance, estimated cumulated mean dose spared was 0.65 Gy, range 0.4–1.0 Gy. Weekly dose surveillance identified no overdoses of brainstem or spinal cord and no compromised target volumes. Time consumption per CBCT/week was median 22 min, range 17–38 min. At an average of 4.5 CBCTs surveyed per patient, this corresponds to 100 min total per patient. Since three out of 40 surveilled patients were selected for adaptive replan, the number of patients needed to see to achieve any dose reduction to parotid glands was 13. Thus, the time investment required to identify the three patients was 22 working hours per patient.
Four of the 40 patients in the weekly surveillance group had a mid-course CT due to field of view limitations in CBCT imaging. Compared to the workflow employed in the CT cohort, in which all patients had mid-course CT imaging, this represents a reduction of 36 mid-course CT scans (90%). None of the four mid-course CTs led to adaptive replanning, as the dose distribution remained consistent with the original plan.
Discussion
In the current study, we found that in both the CT and CBCT cohort, no adaptive replans were triggered by mid-course imaging and dose verification in the presence of daily CBCT image guidance and match protocol, managed by RTTs. In other words, if no systematic irregularities were identified by the RTTs during setup, no clinically relevant target underdose or critical OAR overdose was found on mid-course imaging. In this setting, mid-course imaging could safely be omitted. For the CT-based workflow, this would also mean freeing up timeslots on the CT scanner and sparing patients from unnecessary additional examinations.
In the CBCT group, intensified surveillance with weekly dose verification for a subset of patients identified three patients with increasing mean dose to parotid glands, who would have gone unnoticed through treatment in the CT-based adaptive workflow. Adaptive replanning of these patients yielded only a minimal and hardly clinically relevant benefit in cumulated parotid gland dose and at a high cost in workload. One reason for the limited benefit in spared cumulated dose was that all three patients were identified relatively late in the treatment course, at fractions 18, 20 and 21. Taking into account a few workdays to acquire a rescan and build a new plan, only about a third of the treatment course could be delivered with the improved, adaptive plans. Thus, even with a fair improvement in daily dose delivered, there were too few treatment fractions remaining for this to have much impact on the cumulative dose.
Previous studies have found varying outcomes of ART. In a prospective clinical study, Schwartz et al. found that one adaptive replan in 22 patients reduced ipsilateral and contralateral parotid gland mean dose by 0.6 and 1.3 Gy, respectively, similar to the results in the present study. In a subset of eight patients with two adaptive replans mean doses were further reduced by 0.8 and 4.1 Gy [Citation12]. A recent in silico study by Castelli et al. found a parotid gland mean dose decrease of 3.6 Gy compared to cumulative dose with six weekly replannings in 15 oropharyngeal cancer patients [Citation24]. From the same group, a study by Zhang et al. showed that 95% of this benefit could be achieved by three weekly replannings at the first, second and fifth week of treatment, however they still found a mean benefit of 2.2 Gy with a single adaptive replan [Citation25]. One possible explanation for these differences could be a higher degree of patient selection in these studies, which focused on locally advanced oropharyngeal cancer. In the present study however, the majority of patients completed treatment without the need for adaptive replan.
The low number of replannings triggered by weekly CBCT dose surveillance in this study is due to the high standards of IGRT performed daily with match protocol by the RTTs. This workflow ensured that early anatomical changes such as changes in neck diameter, a relevant factor for parotid gland dose [Citation26], would be identified quickly and adaptive replanning can be initiated.
This study did identify a small number of patients with parotid gland overdose, who would not previously have been identified, even with the match protocol and daily image guidance. The benefit however, was small compared to the workload in this particular study. Future innovations in scripting automated workflows and automated treatment planning might substantially reduce physician and physicist time consumption associated with ART [Citation27], although the benefits shown in this study alone cannot justify investment in such solutions.
The present study was non-randomized and only three events occurred in the weekly dose surveillance group. Thus, no patient or tumor characteristics predicting benefit of surveillance could be identified. The study also included a wide range of tumor types, which may have diluted the results. However, even narrowing down to locally advanced oropharyngeal cancers would require close to 10 working hours to achieve the same small benefit in parotid gland dose.
In conclusion, the tested dose surveillance algorithm resulted in a minimal reduction of dose (< 1 Gy) to the parotid glands for only three of 40 patients at a high cost in working hours. The proposed algorithm is thus not sustainable. In the presence of daily CBCT image guidance, mid-course CT does not provide any added benefit and can safely be omitted. A daily image guidance workflow and match protocol managed by skilled RTTs is a safe and efficient method for identifying patients in need of adaptive replanning.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Jensen K, Jensen AB, Grau C. A cross sectional quality of life study of 116 recurrence free head and neck cancer patients. The first use of EORTC H&N35 in Danish. Acta Oncol. 2006;45:28–37.
- Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: frequency, intensity and correlation with dose and volume parameters. Radiother Oncol. 2007;85:74–82.
- Mortensen HR, Overgaard J, Specht L, et al. Prevalence and peak incidence of acute and late normal tissue morbidity in the DAHANCA 6&7 randomised trial with accelerated radiotherapy for head and neck cancer. Radiother Oncol. 2012;103:69–75.
- Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587.
- Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136.
- Barker JL, Garden AS, Ang KK, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59:960–970.
- Castadot P, Lee JA, Geets X, et al. Adaptive radiotherapy of head and neck cancer. Semin Radiat Oncol. 2010;20:84–93.
- O'Daniel JC, Garden AS, Schwartz DL, et al. Parotid gland dose in intensity-modulated radiotherapy for head and neck cancer: is what you plan what you get? Int J Radiat Oncol Biol Phys. 2007;69:1290–1296.
- Den RB, Doemer A, Kubicek G, et al. Daily image guidance with cone-beam computed tomography for head-and-neck cancer intensity-modulated radiotherapy: a prospective study. Int J Radiat Oncol Biol Phys. 2010;76:1353–1359.
- Hansen EK, Bucci MK, Quivey JM, et al. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64:355–362.
- Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83:986–993.
- Schwartz DL, Garden AS, Shah SJ, et al. Adaptive radiotherapy for head and neck cancer-dosimetric results from a prospective clinical trial. Radiother Oncol. 2013;106:80–84.
- Brouwer CL, Steenbakkers RJ, Langendijk JA, et al. Identifying patients who may benefit from adaptive radiotherapy: does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol. 2015;115:285–294.
- Li H, Zhu XR, Zhang L, et al. Comparison of 2D radiographic images and 3D cone beam computed tomography for positioning head-and-neck radiotherapy patients. Int J Radiat Oncol Biol Phys. 2008;71:916–925.
- Zeidan OA, Langen KM, Meeks SL, et al. Evaluation of image-guidance protocols in the treatment of head and neck cancers. Int J Radiat Oncol Biol Phys. 2007;67:670–677.
- Elstrøm UV, Muren LP, Petersen JB, et al. Evaluation of image quality for different kV cone-beam CT acquisition and reconstruction methods in the head and neck region. Acta Oncol. 2011;50:908–917.
- Hvid CA, Elstrøm UV, Jensen K, et al. Accuracy of software-assisted contour propagation from planning CT to cone beam CT in head and neck radiotherapy. Acta Oncol. 2016;55:1324–1330.
- Yang Y, Schreibmann E, Li T, et al. Evaluation of on-board kV cone beam CT (CBCT)-based dose calculation. Phys Med Biol. 2007;52:685–705.
- van Zijtveld M, Dirkx M, Heijmen B. Correction of conebeam CT values using a planning CT for derivation of the “dose of the day”. Radiother Oncol. 2007;85:195–200.
- Grégoire V, Ang K, Budach W, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110:172–181.
- Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58–S63.
- Dijkema T, Raaijmakers CP, Ten Haken RK, et al. Parotid gland function after radiotherapy: the combined michigan and utrecht experience. Int J Radiat Oncol Biol Phys. 2010;78:449–453.
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–S19.
- Castelli J, Simon A, Louvel G, et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat Oncol. 2015;10:6.
- Zhang P, Simon A, Rigaud B, et al. Optimal adaptive IMRT strategy to spare the parotid glands in oropharyngeal cancer. Radiother Oncol. 2016;120:41–47.
- Capelle L, Mackenzie M, Field C, et al. Adaptive radiotherapy using helical tomotherapy for head and neck cancer in definitive and postoperative settings: initial results. Clin Oncol (R Coll Radiol). 2012;24:208–215.
- Hazell I, Bzdusek K, Kumar P, et al. Automatic planning of head and neck treatment plans. J Appl Clin Med Phys. 2016;17:272–282.
