Abstract
Background: The Copenhagen Breast Cancer Trial (CBCT) randomly assigned patients with early breast cancer to two years of tamoxifen or placebo and we evaluated the effect over the following four decades.
Patient and methods: Between 1975 and 1978, 327 patients with primary breast cancer were randomly assigned to two years of daily placebo or tamoxifen. Survival statistics was collected from the Danish Civil Registration System.
Results: The five-year invasive breast cancer recurrence (BCR) rate was 43.2% in the placebo arm and 31.9% in the tamoxifen arm. Compared with the placebo arm the hazard ratio for a BCR event was 0.73 in the tamoxifen arm (p = .07). With an estimated median follow-up on overall survival of 40.9 years, 154 and 145 patients had died in the placebo and tamoxifen arm, respectively. After adjustment for baseline characteristics a significant reduction in mortality was obtained from tamoxifen (HR 0.79; p = .04).
Conclusion: Two years of adjuvant tamoxifen resulted in a sustained reduction in mortality in pre- and postmenopausal high-risk breast cancer patients with long-term follow-up data.
Introduction
In 1975, when the Copenhagen Breast Cancer Trial (CBCT) was initiated, the clinical and experimental experience of tamoxifen was rather limited. An association between estrogen receptor content and endocrine treatment effect was suggested in 1971 and the first report on the clinical effect of tamoxifen in advanced breast cancer was published the same year [Citation1,Citation2]. No published data from the adjuvant setting was accessible and just one other adjuvant tamoxifen trial was being initiated by McGuire and colleagues [Citation3]. Several additional trials evaluating tamoxifen as an adjunct to local treatment of early breast cancer were launched in the late seventies including the DBCG 77C, NSABP B-09, and NATO trials [Citation4–6]. The benefits of tamoxifen in the adjuvant setting was first reviewed by Mouridsen and Palshof in 1983 and later confirmed at the Consensus Development Conference in 1985 as well as the first EBCTCG meta-analysis published in 1988 [Citation7–9].
Tamoxifen has been shown to reduce the risk of recurrence beyond the duration of treatment and there is evidence supporting that this ‘carry-over’ benefit persists for at least 10 years after completion of 5 years of tamoxifen [Citation10–12]. Moreover, a carry-over benefit persisting beyond 25 years has been suggested following only one or two years of tamoxifen [Citation13,Citation14]. The aim of the this study was to follow the effect of two years of tamoxifen over 40 years which was expected to be the life span for most patients who were postmenopausal at diagnosis.
Patient and methods
The Copenhagen Breast Cancer Trial (CBCT), was a double-blind placebo-controlled phase-3 randomized trial. Identical looking tamoxifen and placebo tablets were provided by Imperial Chemical Industries (ICI), the sponsor of the trial. Patients were randomized to tamoxifen (10 mg) or identical looking placebo and both were administered three times daily. Randomization was stratified according to menopausal status. The organization of CBCT has previously been described in detail [Citation15].
Patients
CBCT included women who achieved complete resection of an invasive unilateral adenocarcinoma of the breast by simple mastectomy (without axillary dissection) and subsequently received adjuvant radiotherapy to the chest wall and regional lymph nodes (40.92 Gy in 22 fractions, 5 fractions per week) as specified in the preceding CBCT trial [Citation16]. Patients were required to be without evidence of advanced disease by physical examination, radiography of the chest and bone scintigraphy and without previous or concomitant thromboembolic disease, chronic hepatitis, clinically significant or untreated hypertension or heart disease and malignant disease.
Estrogen receptor status
The ER content of the primary tumor was measured in histologically verified tumor tissue by a dextran-coated charcoal assay in a single laboratory (Johan Daehnfeldt). Tumors with at least 10 fmol/mg cytosol protein were classified as ER positive. A later re-evaluation revealed a suboptimal sensitivity and tumors originally classified as ER negative might have been weakly ER positive [Citation17].
Follow-up
Treatment-related adverse events and findings on clinical and laboratory examination were recorded every 3 months the first two years and then every 6 months for the next three years. Bone scintigraphy was done yearly and radiography of the chest and contralateral breast was done every 18 months. The Danish Civil Registration System (CRS) contains continuously updated information on vital status and emigrations and through linkage to CRS by each participant’s unique civic registration number a complete follow-up until 1 August 2017 was retrieved on survival [Citation18].
Statistical analysis
The statistical office of the DBCG undertook a central review and analysis of data. The primary endpoint of the original study was time to breast cancer recurrence (BCR) defined as time from randomization to any first event of invasive ipsi- or contralateral breast recurrence, local or regional invasive recurrence or distant recurrence. As inclusion in this study was completed 39 years ago, we as the primary endpoint chose overall survival (OS) defined as time from randomization to death irrespective of cause of death. Follow-up time was quantified in terms of a Kaplan–Meier estimate of potential follow-up [Citation19].
OS was analyzed unadjusted by the Kaplan–Meier method and groups were compared using the log-rank test. Cumulative incidence of BCR was analyzed with death without recurrence as competing event. Expected survival was calculated by applying age and calendar year specific female mortality figures of the general Danish population to the corresponding person-years of the patient cohort. Hazard ratios [HR] were estimated from the Cox proportional hazards regression model (OS) and the Fine-Gray proportional hazards subdistribution model (BCR) to quantify the effect of treatment regimen and to explore interactions. Interactions between treatment and the covariates were investigated in separate models. The assumptions of proportional hazards were assessed by Schoenfeld residuals, and by including a time-dependent component in the model. Associations between treatment regimen and other characteristics were analyzed by chi-square. p values are two-tailed. Statistical analyses were done with the statistical software SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Ethics and role of sponsor
The funders designed the study and were responsible for data collection. The DBCG statistical office was responsible for linking to the CRS, the final decision regarding manuscript content and submission. Placebo or tamoxifen (Nolvadex®) 10 mg (Imperial Chemical Industries (ICI), UK) was taken orally, three times daily continuously for two years.
Results
CBCT was open from March 1975 through March 1978, and this analysis was conducted 39 years after closure of recruitment. Median estimated potential follow-up was 6.3 years for BCR and 40.9 years for OS. Among 317 participants, 164 were randomized to tamoxifen and 153 to placebo, and patient characteristics are shown in . Information on ER content was available from 196 patients and 127 (65%) were classified as ER positive.
Table 1. Patient and tumor characteristics.
A total of 133 events of local- or distant BCR were observed during the clinical follow-up and shows the cumulative incidence curves for BCR. In the intent to treat (ITT) analysis (N = 317), the overall unadjusted HR for BCR in the tamoxifen group compared with the placebo group was 0.73; 95% confidence interval [CI] 0.52 to 1.02; p = .07. When adjusting for baseline characteristics, including ER, stage and menopausal status, the benefit of tamoxifen remained unchanged (adjusted HR 0.74; CI 0.53–1.05; p = .09). BCR remained largely unchanged following exclusion of ER-negative patients (adjusted HR 0.72; CI 0.49–1.08), and shows the CI-curves for patients with ER positive or unknown tumors.
Figure 1. Panel A shows cumulative incidence for Breast Cancer Recurrence (BCR) of the 317 patients included in the intent to treat analysis who were randomly allocated to tamoxifen or placebo. Panel B shows the estimates in patients with confirmed ER positive or ER unknown tumors. Panel C shows the Kaplan–Meier estimates of overall survival of the 317 patients included in the intent to treat analysis who were randomly allocated to tamoxifen or placebo. The gray curves show the expected survival for the 317 patients, applying mortality figures of the general Danish population. Panel D shows the Kaplan–Meier estimates of overall survival in patients with confirmed ER positive or ER unknown tumors. Number of patients at risk are given below x-axes.
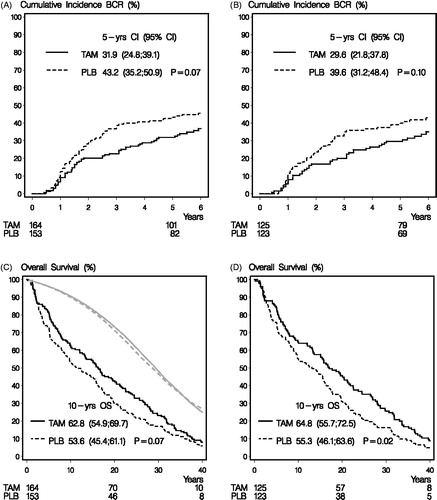
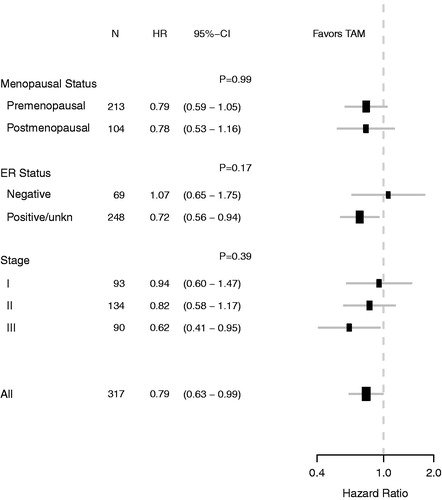
By data cutoff, 299 (94%) of the 317 patients had died and 154 and 145 deaths occurred in the placebo and tamoxifen group, respectively. The bottom lines in shows a trend towards a reduction in survival from tamoxifen (HR 0.81; CI 0.65–1.07; p = .07). After adjustment for baseline characteristics, a significant survival benefit was obtained from tamoxifen in the ITT population (adjusted HR 0.79, CI 0.63–0.99; p = .04). For comparison, the survival is in the two upper curves shown for age-matched women in the entire Danish population (). The effect of tamoxifen remained largely unchanged by the exclusion of ER negative patients (adjusted HR 0.72; CI 0.56–0.94) as shown in . As shown in , we found no statistical evidence of heterogeneity on the effect of tamoxifen from menopausal status, ER status or stage.
Figure 2. The forest plot illustrates exploratory subgroup analysis of overall survival according to menopausal status, ER status and Stage. Hazard ratios (HRs) refer to adjusted estimates obtained in the multivariate analysis of the intent-to-treat population. CI indicates 95% confidence interval. p-values are for test of heterogeneity of treatment effect.
Discussion
This is the longest follow-up ever of an adjuvant tamoxifen trial, and the sustained reduction in mortality from two years of tamoxifen shown in this 40-year analysis of the Copenhagen Breast Cancer Trial has important implications. In particular the long-lasting survival benefit from two years of tamoxifen confirms that patients with pronounced side effects from endocrine treatment can be reassured that even a couple of years of treatment are worthwhile. The reduction in mortality appeared in pre- and postmenopausal patients but may not include patients with ER negative tumors.
This study has several strengths, including its placebo-controlled design with provision of study drugs free of charge. Moreover the importance of achieving local control of breast cancer was already well recognized in the seventies, and as a result of the first Copenhagen breast cancer trial, patients in this study received a mastectomy followed by radiotherapy [Citation16,Citation20]. At the time of enrollment into CBCT patients already had been assigned a unique 10-digit civic registration number, and we obtained a complete follow-up on survival from the CRS who ever since on a daily basis has updated information on migration and vital status [Citation18].
CBCT only included 317 patients and the small sample size limited our ability to evaluate the benefit from tamoxifen in subgroups. Several other limitations should be taken into account when interpretation the results of this study. First, the clinical work-up only continued for eight years from inclusion of the first patient leaving a somewhat short follow-up for breast recurrences. Second, tamoxifen was in CBCT only given for two years and an incremental benefit would be achievable by continuation for five or even 10 years. Third, a further benefit can be achieved in postmenopausal women from substituting tamoxifen with an aromatase inhibitor or giving tamoxifen and an aromatase inhibitor in a sequence [Citation21,Citation22]. Fourth, tissue specimens for hormone receptor assays were not available from about 40% of the patients randomly assigned to treatment in CBCT. By todays standard a low availability, but it must be taken into account that the possibilities to predict anti-estrogen treatment only was hypothesized when the CBCT began [Citation23]. Fifth, the standards for the biochemical hormone receptor test used in this study was revised along the way and finalized after recruitment was completed [Citation24,Citation25]. Finally, long term and close to lifelong follow-up is inevitably associated with non-proportional hazards. A closer investigation of the carryover effect could potential be possible by dividing the follow-up period in intervals, but this is in the current study prohibited by the small sample size of our study [Citation26].
In conclusion, two years of tamoxifen improves outcome in high-risk breast cancer patients. The impact on mortality is long-lasting implying that tamoxifen possess cytotoxic ability. This study confirms that the same degree of benefit seems to be achieved in pre- as well as in postmenopausal breast cancer patients but the benefit may be restricted to patients with estrogen receptor positive breast cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jensen EV, Block GE, Smith S, et al. Estrogen receptors and breast cancer response to adrenalectomy. Natl Cancer Inst Monogr. 1971;34:55–70.
- Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25:270–275.
- Hubay CA, Pearson OH, Marshall JS, et al. Adjuvant chemotherapy, antiestrogen therapy and immunotherapy for stage II breast cancer: 45-month follow-up of a prospective, randomized clinical trial. Cancer. 1980;46(12 Suppl):2805–2808.
- Fisher B, Redmond C, Brown A, et al. Treatment of primary breast cancer with chemotherapy and tamoxifen. N Engl J Med. 1981;305:1–6.
- Rose C, Thorpe SM, Mouridsen HT, et al. Antiestrogen treatment of postmenopausal women with primary high risk breast cancer. Breast Cancer Res Tr. 1983;3:77–84.
- Baum M, Brinkley DM, Dossett JA, et al. Improved survival among patients treated with adjuvant tamoxifen after mastectomy for early breast cancer. Lancet. 1983;2:450.
- Mouridsen HT, Palshof T. Adjuvant systemic therapy in breast cancer; a review. Eur J Cancer Clin Oncol. 1983;19:1753–1770.
- Consensus conference. Adjuvant chemotherapy for breast cancer. JAMA. 1985;254:3461–3463.
- Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681–1692.
- Stewart HJ, Prescott RJ, Forrest AP. Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. J Natl Cancer Inst. 2001;93:456–462.
- Rutqvist LE, Johansson H. Stockholm Breast Cancer Study Group. Long-term follow-up of the randomized Stockholm trial on adjuvant tamoxifen among postmenopausal patients with early stage breast cancer. Acta Oncol. 2007;46:133–145.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784.
- Knoop AS, Laenkholm AV, Jensen MB, Danish Breast Cancer Cooperative Group, et al. Estrogen receptor, Progesterone receptor, HER2 status and Ki67 index and responsiveness to adjuvant tamoxifen in postmenopausal high-risk breast cancer patients enrolled in the DBCG 77C trial. Eur J Cancer. 2014;50:1412–1421.
- Ekholm M, Bendahl PO, Fernö M, et al. Two years of adjuvant tamoxifen provides a survival benefit compared with no systemic treatment in premenopausal patients with primary breast cancer: long-term follow-up (>25 years) of the Phase III SBII:2pre trial. JCO. 2016;34:2232–2238.
- Palshof T, Mouridsen HT, Daehnfeldt JL. Adjuvant endocrine therapy of primary operable breast cancer. Report on the Copenhagen breast cancer trial. In: Mouridsen H T and Palshof T, editors. Breast cancer–experimental and clinical aspects. Oxford (UK): Pergamon Press; 1979. p. 183–8.
- Kaae S, Johansen H. Simple mastectomy plus postoperative irradiation by the method of McWhirter for mammary carcinoma. Ann Surg. 1969;170:895–899.
- Thorpe SM, Poulsen HS, Pedersen KO, et al. Quality control of receptor analyses of breast cancer tissue in Denmark. Acta Oncol. 1988;27:621–625.
- Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549.
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346.
- EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135.
- BIG 1-98 Collaborative Group, Mouridsen H, Giobbie-Hurder A, Goldhirsch A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:766–776.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352.
- Horwitz KB, McGuire WL, Pearson OH, et al. Predicting response to endocrine therapy in human breast cancer: a hypothesis. Science. 1975;189:726–727.
- EORTC Breast Cancer Co-operative Group. Revision of the standards for the assessment of hormone receptors in human breast cancer; report of the second E.O.R.T.C. Workshop, held on 16-17 March, 1979, in the Netherlands cancer institute. Eur J Cancer. 1980;16:1513–1515.
- Koenders A, Thorpe SM. Standardization of steroid receptor assays in human breast cancer–I. Reproducibility of estradiol and progesterone receptor assays. Eur J Cancer Clin Oncol. 1983;19:1221–1229.
- Dignam JJ, Dukic V, Anderson SJ, et al. Hazard of recurrence and adjuvant treatment effects over time in lymph node-negative breast cancer. Breast Cancer Res Treat. 2009;116:595–602.
