Abstract
Background: The prognosis of breast cancer has improved significantly during the last few decades increasing the interest in health-related quality of life (HRQoL). The aim of this study was to compare the HRQoL scores produced by different instruments and to shed light on their validity in various states of breast cancer by studying the association of cancer-related symptoms with HRQoL.
Material and methods: An observational, cross-sectional study of breast cancer patients treated in the Helsinki and Uusimaa Hospital District from September 2009 to April 2011. A total of 840 patients completed three HRQoL questionnaires: the EQ-5D-3L (including VAS), 15D and EORTC QLQ-30 and a questionnaire concerning sociodemographic factors. Patients were divided into five mutually exclusive groups: primary treatment (n = 118), recovery (6–18 months from diagnosis) (n = 150), remission (>18 months) (n = 382), metastatic disease (n = 176) and palliative care (n = 14). The association of HRQoL with sociodemographic and clinical factors and cancer-related symptoms, screened by the EORTC QLQ-30, was studied by multivariate modeling using stepwise linear regression analysis.
Results: HRQoL scores were the best at the time closest to diagnosis and deteriorated with disease progression. The EQ-5D had a pronounced ceiling effect with 40.8% of the respondents scoring 1 (perfect health) compared to 6% for the 15D and 5.6% for VAS. In regression analyses, pain, fatigue and financial difficulties were the most important predictors of lower HRQoL. The 15D showed better discriminatory power and content validity. The EORTC QLQ-C30 functioning deteriorated in advanced states of the disease with physical, social and role functioning being the most affected. Insomnia, fatigue and pain were the most commonly reported symptoms in all groups.
Conclusions: Different HRQoL instruments produce notably different HRQoL scores. The EQ-5D has a pronounced ceiling effect. Pain and fatigue are the most common symptoms associated with poor HRQoL in all disease states.
Introduction
Breast cancer is the most frequent cancer after lung cancer and the most frequent cancer among women worldwide [Citation1]. In Finland (population 5.5 million), the numbers of breast cancer patients alive were 67.010 women and 244 men in 2015 [Citation2]. The latest incidence statistics showed 5008 new breast cancer cases among women in 2014 and also in Finland, the incidence has been predicted to rise [Citation2–4].
Effectiveness and safety are the main drivers of treatments, but recently emphasis has also been focused on patient-experienced health-related quality of life (HRQoL) [Citation5–9]. Placing emphasis on factors that affect HRQoL is of both humane and economic importance [Citation10]. HRQoL can be assessed with both generic and disease-specific HRQoL instruments and their usage varies in different studies [Citation11–14]. In an attempt to produce better results of care and more effective treatments, more emphasis has been put on the research on HRQoL [Citation10]. HRQoL varies among different states of breast cancer and numerous factors influence it [Citation11,Citation15]. It is usually better at the time of diagnosis but deteriorates as the disease progresses [Citation16]. The need for studies providing more information on HRQoL at different states of breast cancer has been pointed out [Citation13].
The aim of this study was to assess the HRQoL of breast cancer patients, to compare the HRQoL scores produced by different HRQoL instruments and to shed light on their validity in various states of breast cancer by studying the association of cancer-related symptoms, screened by the EORTC QLQ-30, with HRQoL.
Patients and methods
Patients
Patients with histologically verified breast cancer were identified from hospital records and asked to participate in this observational and cross-sectional study between September 2009 and April 2011 in the Helsinki and Uusimaa Hospital District. Patients were recruited when coming to an appointment to the hospital, or by sending them an invitation by mail. Patients were asked to fill in the study questionnaires. One reminder was sent if there was no response. The study was approved by the Ethics Committee of the Helsinki and Uusimaa Hospital District (Permission 68(11.06.2008,207/13/03/02/08)). All patients gave a written informed consent.
Sociodemographic and medical information
The data that were collected from the patients included three different questionnaires: EQ-5D, 15D and EORTC. Patients also filled in a questionnaire concerning demographic background questions such as education and marital and occupational status. Specific data concerning the type of cancer and treatment given were collected from hospital records.
Health-related quality of life assessment
Two generic HRQoL instruments, the 15D and the three-level version of the EQ-5D (EQ-5D-3L, including a visual analog scale, VAS), as well as the cancer-specific EORTC QLQ-C30 questionnaires, were used. All instruments are standardized and self-administered [Citation17–19].
The 15D can be used both as a profile and a single index score measure (utility score, which is required for calculation of quality-adjusted life years). The health state descriptive system (questionnaire) is composed of the following dimensions: mobility, vision, hearing, breathing, sleeping, eating, speech (communication), excretion, usual activities, mental function, discomfort and symptoms, depression, distress, vitality and sexual activity. For each dimension, the respondent chooses one of the five ordinal levels best describing his/her state of health at the moment. The single index score (15D score) represents the overall HRQoL and ranges from 0 to 1 (1 = full health, 0 = being dead) [Citation17]. The minimum clinically important change or difference (MID) in the 15D score is 0.015 [Citation20].
The EQ-5D also produces a utility score (EQ-5D score), but no health profile. In the EQ-5D, the respondent chooses from the five different dimensions an ordinal level from 1 to 3 best describing his/her health. In addition, the questionnaire includes a VAS. To calculate the EQ-5D score, we used the UK time-trade-off (TTO) tariff. The EQ-5D score can range from –0.594 to 1.000 and the EQ-5D VAS from 0 to 100 (mm in visual scale) [Citation12,Citation19]. The minimum clinically important change or difference (MID) in the EQ-5D score is 0.08 and in the VAS 7-12 [Citation21].
The EORTC QLQ-C30 is a cancer-specific HRQoL questionnaire developed to detect disease- and treatment-specific health problems. It has been translated into more than 100 different languages and used in hundreds of studies indicating that it is a valid disease-specific HRQoL measure in cancer. It measures functional ability and symptoms on a 1–4 scale and also includes the respondent’s overall rating of his/her quality of life and health during the last week on a 1–7 scale. EORTC does not produce a utility score. The functionalities measured are physical, role, social, emotional and cognitive functioning. The symptoms measured are fatigue, nausea/vomiting and pain and the symptoms defined constipation, diarrhea, appetite loss, insomnia, dyspnea and financial difficulties [Citation18].
Statistical analysis
We formed five mutually exclusive groups based on the state of cancer: primary treatment, recovery (6–18 months from diagnosis), remission (>18 months from diagnosis), metastatic disease and palliative care (after life-prolonging, oncological treatments were terminated).
Descriptive statistics were calculated for all the groups and are given as percentages, means and standard deviations (SD). Patient characteristics are shown in .
Table 1. Patient characteristics.
The association of clinical and demographic factors as well as the EORTC symptom and functioning scale scores with the 15D, EQ-5D and VAS scores were explored using linear, stepwise regression analysis. In phase 1 analysis, clinical and demographic factors were entered as potential explanatory variables including the disease states. In phase 2, the EORTC symptom and functioning scale scores were entered as potential explanatory variables in addition to variables which turned out statistically significant in phase 1. It was hypothesized that the higher the R2, i.e., the share of the variance of the scores of instrument X explained by EORTC (criterion), the more X has common variance and content with it and the more valid X is in terms of content and criterion validity in this patient group. The statistical significance of differences between the groups in the means was tested with independent samples t-test.
The sensitivity of the instruments in terms of discriminatory power, i.e., ability to detect differences in health status cross-sectionally, is measured by the ceiling and floor effects, i.e., the percentage of patients obtaining a maximum and minimum score, respectively. The lower the percentages, the better the instrument is in terms of discriminatory power. We also hypothesize that a high ceiling effect in a serious disease like cancer, and especially in the a priori severe metastatic and palliative states, undermines the instrument’s credibility and validity in this patient group. p values ≤.05 were considered statistically significant. The analyses were performed using SPSS software version 22 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Out of the 1424 invited patients, 840 patients participated in the study (response rate 59%). Five were male (0.6%). The age of the patients varied between 26 and 90 years with a mean of 61.7 years.
The majority of the patients had hormone receptor-positive disease: 84% had estrogen hormone receptors and 64% progesterone receptors. HER-2-oncogene expression was detected in 20% of all patients.
The demographic factors of patients, the tumor characteristics and the treatments given during the three months preceding the survey, are shown in . Altogether, 190 (23% of all) patients had distant metastases.
Health-related quality of life
The analysis showed differences in the mean scores across various disease states. The mean scores were higher in the primary treatment group and lower as the disease progressed. The growing symptom burden at metastatic states was reflected in poorer HRQoL.
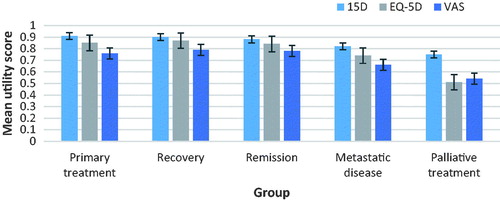
Comparing the results obtained with different instruments showed some similarities and some notable differences ( and ). There was no statistically significant and clinically important difference in the HRQoL scores between the groups of primary treatment and recovery with any of the instruments. For the 15D score, the mean difference between the groups of primary treatment and remission was both statically significant and clinically important, but this was not the case for the other two instruments. The mean scores for all states were higher with the 15D than with the EQ-5D. VAS followed the same trend; mean scores were lower than with the other instruments in all but the palliative care group. For this group, the EQ-5D gave the lowest mean scores.
Table 2. Mean HRQoL scores in different disease states as measured with different instruments.
The EQ-5D had a pronounced ceiling effect: 343 of 830 patients, i.e., 41.3% obtained a score of 1 (full health), as compared to 6.0% with the 15D (n = 50/835) and 5.6% with the VAS (n = 46/828). Even in the metastatic and palliative states, the EQ-5D had ceiling effects of 26.2% and 8.3%, respectively, whereas the other instruments virtually none.
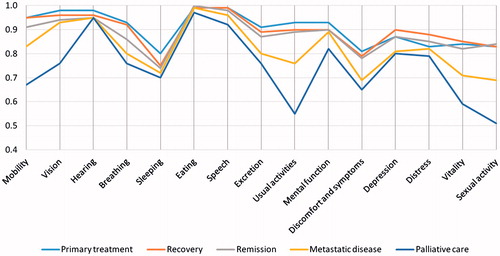
Of the dimensions of the 15D, sleeping and discomfort and symptoms were the most impaired in all disease groups. Usual activities, mobility, vitality and sexual activity were more impaired in the metastatic disease state and in the palliative care group ().
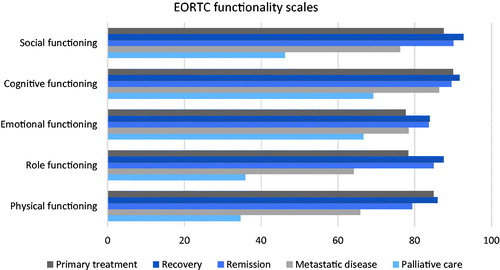
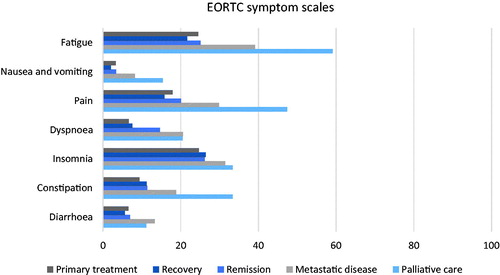
The EORTC QLQ-C30 functioning deteriorated in advanced states of the disease with physical, social and role functioning being the most affected (). Insomnia, fatigue and pain were the most commonly reported symptoms in all groups. The symptom burden was highest in patients with advanced disease, especially in the palliative care group ().
Factors influencing HRQoL
Results of the linear regression analyses are shown in . In phase 1 analysis, the clinical and demographic factors explained 26–38% of the variance in the HRQoL scores. Financial difficulties, higher age and belonging to the metastatic or palliative care group were associated with poorer HRQoL. When symptom and functionality scales of the EORTC were included in the phase 2 analysis, the explanatory power increased to 72.5% for the 15D, 63.1% for the EQ-5D and 52.2 for VAS. Financial difficulties, fatigue and pain impaired HRQoL measured by any of the instruments.
Table 3. Statistically significant factors influencing HRQoL scores, results of the regression analyses.
Discussion
We observed that different HRQoL instruments gave remarkably different values. However, regardless of the instrument used, the HRQoL varied a lot in different states of the disease and deteriorated in line with the increased symptom burden and functional deterioration in advanced states of the disease.
Comparison of the instruments
Breast cancer patients’ HRQoL is a complex matter and the choice of HRQoL instrument is demanding [Citation13]. Different instruments give different types of information, which makes the comparison of various studies challenging [Citation14,Citation16]. A systematic review of HRQoL of long-term (more than 5 years since diagnosis) breast cancer survivors addressed different instruments, both generic and disease-specific, including the EORTC QLQ-C30 questionnaire, which was also used in this study [Citation13]. The review pointed out a need for a study like ours taking into account the effect of the disease state and the importance of psychosocial aspects and the need for including different treatments in the analysis when studying HRQoL of breast cancer patients. The instrument to be used should be carefully selected bearing in mind these aspects [Citation13]. We discovered that the patients’ assessments of their HRQoL based simply on VAS were poorer than what the two HRQoL instruments used suggested. This might be relevant in measuring HRQoL, especially in symptomatic advanced disease. The negative effect of disease progression on HRQoL was well demonstrated with all instruments used. The EQ-5D showed a remarkable ceiling effect across all disease states, even the most advanced ones, where one would not expect scores indicating full health. This casts doubt on the discriminatory power, credibility and thus the validity of the EQ-5D in this patient group. Therefore, the instruments to be used for measuring HRQoL in breast cancer patients should be carefully selected not to lose relevant information.
The effect of the disease state
Individual expectations of life in general and of how the disease will affect one’s life influence the experience of HRQoL [Citation11]. In this study, at the time of diagnosis, the majority of patients had no metastases and little symptoms. After breast cancer diagnosis, the treatments, like surgery, radiation therapy and chemotherapy followed by targeted and/or hormonal therapy, can cause different types of side effects and thus impair HRQoL [Citation22]. However, there were no statistically significant differences in the mean HRQoL scores from primary treatment to recovery for any of the instruments. In the remission and metastatic states, the mean HRQoL scores differed from those at baseline in a statistically significant manner. The significant deterioration of HRQoL with metastatic disease, and especially in the palliative phase of the disease, can be explained with progressive functional deterioration and increased symptom burden. Pain and fatigue were most commonly reported in advanced cancer. They are both activity-limiting symptoms and thus further impair activities of daily living and HRQoL. Emotional and cognitive functions were better preserved despite of the disease progression. For example, insomnia was similarly reported by patients in an early or advanced state of the disease. According to previous studies, emotional functioning of cancer patients is, however, poorer than what is reported in population-based studies [Citation23]. This was more clearly apparent for younger patients [Citation22]. It seems that cancer diagnosis itself causes psychosocial distress throughout the disease trajectory [Citation23].
Factors influencing HRQoL
In line with our findings, pain and sleeping disorders are, according to a systematic review, the symptoms affecting cancer survivors’ HRQoL the most [Citation13]. Fatigue and depression were particularly impairing factors of HRQoL of breast cancer patients shortly after the termination of cancer treatments, and it has been implied that physical activity is a positive factor regarding breast cancer patients’ HRQoL, especially for those with symptoms like fatigue and depression [Citation24]. In a study of breast cancer patients with advanced disease, pain, depression and poor physical performance status were the leading factors impairing the HRQoL [Citation25]. In the present study, fatigue, pain and financial difficulties were the major factors negatively influencing the HRQoL regardless of the disease state. Screening and treating effectively these activity-limiting symptoms could improve functioning in daily life and thus improve HRQoL.
In our study, age seemed to impair HRQoL, which is in line with that found in the general population. Older patients usually have a poorer quality of life – but in cancer patients, this may be different. Literature namely indicates that in cancer patients, at least in the early stages of the disease, HRQoL is more impaired in younger patients [Citation22,Citation26]. Our study population included all different states of the disease, which might explain the lack of the negative effect of age on HRQoL in our material.
As limitations of our study can be seen the modest response rate of 59% and the fact that the study was cross-sectional with no follow-up. The strength of this study is that it covers all different states of breast cancer with multiple of HRQoL questionnaires used. The results are in line with those of previous studies and the ceiling effect of the EQ-5D was remarkable also in this study.
Summary
In our study, different instruments gave different HRQoL results. VAS gave the lowest scores and EQ-5D had the clearly highest ceiling effects across all states of the disease. The HRQoL deteriorated alongside with the disease progression, especially physical, social and role function were affected. In all disease states activity-limiting symptoms, like pain and fatigue, along with financial difficulties impaired HRQoL. Systematic symptom assessment and treatment could improve activities in daily living and HRQoL. Functions and symptoms screened by the EORTC explained a clearly higher share of the variance of the 15D scores than that of the EQ-5D scores. Thus, the 15D has more common variance and content with the EORTC. If EORTC can be regarded as an established instrument for measuring disease-specific HRQoL in cancer, then the 15D might outperform the EQ-5D in terms of content and criterion validity when measuring generic HRQoL in this patient group.
Acknowledgments
All authors have participated in the writing process of this article. We would like to thank Susanna Helenius, for her help and support in administering the questionnaires to the patients and gathering the clinical data from patient records.
Disclaimer: Harri Sintonen is the developer of the 15D and one of the developers of the EQ-5D.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
Cancer Society of Finland and GlaxoSmithKline Oy, Finland have sponsored the data collection. The study sponsors had no involvement in the study design, collection, analysis, interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr
- Finnish Cancer Registry, Cancer Statistics; [cited 2016 Mar 5]. Available from: www.cancerregistry.fi
- Engholm G, Ferlay J, Christensen N, et al. NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010;49:725–736.
- Engholm G, Ferlay J, Christensen N, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.1 (09.07.2015). Finland: Association of the Nordic Cancer Registries. Danish Cancer Society; [cited 2015 Sep 12]. Available from: http://www.ancr.nu
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241.
- Meretoja TM, Svarvar C, Jahkola TA. Outcome of oncoplastic breast surgery in 90 prospective patients. Am J Surg. 2010;200:224–228.
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012;38:395–398.
- von Smitten K. Margin status after breast-conserving treatment of breast cancer: how much free margin is enough? J Surg Oncol. 2008;98:585–587.
- Editorial. Quality of life and clinical trials. Lancet. 1995;346:1–2.
- Glaser AW, Fraser LK, Corner J, et al. Patient-reported outcomes of cancer survivors in England 1–5 years after diagnosis: a cross-sectional survey. BMJ Open. 2013;3:e002317.
- Carr AJ, Gibson B, Robinson PG. Measuring quality of life: is quality of life determined by expectations or experience? BMJ. 2001;322:1240–1243.
- Brooks R, with the EuroQol Group. EuroQol: the current state of play. Health Policy. 1996;37:53–72.
- Chopra I, Kamal KM. A systematic review of quality of life instruments in long-term cancer survivors. Health Qual Life Outcomes. 2012;10:14.
- Crott R, Versteegh M, Uyl-de-Groot C. An assessment of the external validity of mapping QLQ-C30 to EQ-5D preferences. Qual Life Res. 2013;22:1045–1054.
- Penttinen H, Rautalin M, Roine R, et al. Quality of life of recently treated patients with breast cancer. Anticancer Res. 2014;34:1201–1206.
- Färkkilä N, Torvinen S, Roine RP, et al. Health-related quality of life among breast, prostate and colorectal patients with end-state disease. Qual Life Res. 2014;23:1387–1394.
- Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33:328–336.
- The EORTC Quality of Life Group & EORTC Quality of Life Unit. Guidelines for assessing quality of life in EORTC Clinical Trials. Brussels: EORTC Data Center; 2002. ISBN 2-930064-27-7.
- EuroQoL Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
- Alanne S, Roine RP, Rasanen P, et al. Estimating the minimum important change in the 15D scores. Qual Life Res. 2015;24:599–606.
- Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70.
- Hoyer M, Johansson B, Nordin K, et al. Health-related quality of life among women with breast cancer – a population-based study. Acta Oncol. 2011;50:1015–1026.
- Campbell-Enns H, Woodgate R. The psychosocial experiences of women with breast cancer across the lifespan: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2015;13:112–121.
- Penttinen HM, Saarto T, Kellokumpu-Lehtinen P, et al. Quality of life and physical performance and activity of breast cancer patients after adjuvant treatments. Psycho-Oncology. 2011;20:1211–1220.
- Kokkonen K, Saarto T, Mäkinen T, et al. The functional capacity and quality of life of women with advanced breast cancer. Breast Cancer. 2017;24:128–136.
- Roine E, Blomqvist C, Kellokumpu-Lehtinen PL, et al. Health-related quality of life in breast cancer patients after adjuvant treatments. Breast J. 2016;22:473–475.