Abstract
Purpose: To compare GI symptoms, measures of generic and disease specific health related quality of life (HRQoL), anorectal and pudendal nerve function and anal sphincter morphology between (i) patients ≥2 years after 3D conformal radiotherapy (3D-CRT)±high dose rate (HDR) brachytherapy for carcinoma of the prostate and aged matched patients before radiotherapy and (ii) symptomatic and asymptomatic patients ≥2 years after 3D-CRT ± HDR brachytherapy.
Material and methods: Methodology included: (i) modified LENT-SOMA scales for GI symptoms, (ii) EORTC QLQ-C30 and EORTC QLQ-PR25 questionnaires for generic and disease specific HRQoL, (iii) anorectal manometry and terminal motor latency for anorectal and pudendal nerve function and (iv) endorectal ultrasound for anal sphincter morphology. GI symptoms, parameters of HRQoL, anorectal and pudendal nerve function and anal sphincter morphology were compared using Mann–Whitney’s U, unpaired t and χ2 tests.
Results: Impairment of HRQoL bowel symptoms in the patients ≥2 years after 3D-CRT ± HDR brachytherapy was associated with worse anorectal motor and sensory function, internal and external anal sphincter morphology and 5× greater prevalence of pudendal nerve dysfunction compared with age matched patients before radiotherapy. Symptomatic patients had worse (i) HRQoL measures including global quality of life and bowel and urinary symptom scores, (ii) rectal bleeding, fecal urgency and incontinence scores and (iii) a 2× higher prevalence of pudendal nerve dysfunction compared with asymptomatic patients.
Rectal and anal (i) V 40 Gy >65%, (ii) Dmax >60 Gy, (iii) pudendal nerve Dmax >60 Gy and (iv) Anal V 60 Gy >40% were associated with a greater prevalence of pudendal nerve dysfunction.
Conclusions: 3D-CRT ± HDR brachytherapy for prostate carcinoma, impairs late functional measures including HRQoL, anorectal and pudendal nerve function. Rectal, anal and pudendal nerve radiation dose constraints are proposed for reducing the prevalence of pudendal nerve dysfunction.
Introduction
External beam radiotherapy (EBRT) is preferred for the curative treatment of localized prostate carcinoma by many, particularly older men [Citation1]. The treatment is associated with a 50% reduction in the 10-year prostate cancer mortality compared with endocrine treatment alone [Citation2]. Despite technological advances, gastrointestinal (GI) symptoms such as increased stool frequency, rectal bleeding, urgency and fecal incontinence (FI) occur in the majority of patients during EBRT. These GI symptoms persist in approximately 50% of patients at 5 years [Citation3–5]. Chronic GI symptoms, particularly rectal urgency and FI result in major impairment of the quality of life of these patients [Citation6]. With the exception of rectal bleeding, the ongoing GI symptoms are often refractory to treatment [Citation6,Citation7]. Furthermore, a prospective, randomized trial of EBRT using 3D conformal radiotherapy (3D-CRT) for prostate cancer reported that soiling and FI occurred in over 50% of patients at 3 years [Citation5], suggesting that the prevalence of anorectal dysfunction even with advanced treatment techniques including IMRT have been under-estimated [Citation3,Citation5].
The pathophysiology of anorectal dysfunction after EBRT for carcinoma of the prostate is not fully characterized. Weakness of the external anal sphincter (EAS) and the internal anal sphincter (IAS), decreased rectal compliance, increased rectal sensitivity and faster distal colonic transit have all been implicated [Citation8–10]. However, it remains uncertain whether the underlying etiology of the radiation-induced anorectal dysfunction is predominantly myogenic or neurogenic in nature.
The pelvic nerves and their branches regulate motor, sensory and autonomic activity in the anorectum. The pudendal nerves innervate the EAS and IAS and are vulnerable to damage during radiation treatment of prostate carcinoma. Striated muscle fibers constituting the EAS is considered more resistant to radiation damage compared to neural tissue [Citation8]. There is a paucity of studies examining the role of pudendal nerve injury in radiation-induced anorectal dysfunction.
Our recent data suggest pudendal nerve dysfunction is important in the pathogenesis of FI 3–15 years after EBRT for prostate carcinoma [Citation11]. However, in that study only three of the 17 patients examined were treated with 3D-CRT and radiation dose volume data of organs at risk (OAR) were based on modeling of available CT data only.
The aims of the current study were to further evaluate the etiology of radiation induced anorectal dysfunction by comparing GI symptoms, measures of generic and disease specific health related quality of life (HRQoL), anorectal and pudendal nerve function and anal sphincter morphology between (i) men ≥2 years after 3D-CRT ± high dose rate (HDR) brachytherapy and age matched patients before radiotherapy for carcinoma of the prostate and (ii) symptomatic and asymptomatic patients ≥2 years after 3D-CRT ± HDR brachytherapy for carcinoma of the prostate.
Material and methods
Study population
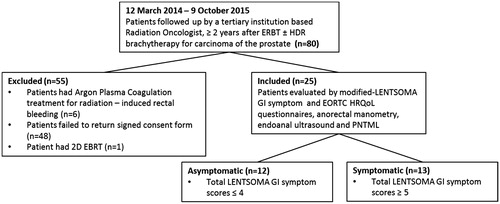
The study population was recruited from among 80 patients still attending follow-up during the period between 12 March 2014 and 9 October 2015 after completing EBRT ± HDR brachytherapy ≥2 years for localized (UICC T1–T3, N0, M0) prostate carcinoma under the care of a tertiary institution based Radiation Oncologist. Eligibility criteria for the study were (i) completion of 3D-CRT ± HDR ≥2 years previously, (ii) no clinical or radiological evidence of relapse, (iii) no therapy likely to influence evaluation of anorectal function such as argon plasma coagulation (APC) therapy for radiation-induced rectal bleeding and/or a constant requirement for anti-diarrheal medication and (iv) signed informed consent to participate in the institutional approved study protocol.
Written information of the study protocol including a consent form previously approved by the institutional research ethics committee was mailed to each patient after being verbally informed of the study protocol in clinic. This was followed by a telephone call two weeks later by one of the graduate scientist research assistants (ADiM). Forty patients declined to participate in the study protocol for the following reasons: (i) feeling unwell from other medical conditions, (ii) not interested, had no time or had concerns about the study protocol (21 including one patient unable to attend because he could not do without loperamide medication for alleged radiation-induced bowel disease) and (iii) transport difficulties [Citation9]. Eight patients who declined the study did not state a reason.
Of the 32 who returned the signed informed consent form, seven patients were excluded due to APC therapy for radiation-induced rectal bleeding (n = 6) or radiotherapy with a 2D technique 15 years previously (n = 1) ().
Of the 25 remaining patients who met all eligibility criteria, 23 received 3D-CRT alone, two had EBRT + HDR brachytherapy ().
Table 1. Study population demographics and radiation treatment characteristics for symptomatic and asymptomatic patients.
Twelve patients were classified asymptomatic and 13 as symptomatic () on the basis of a modified total LENT-SOMA GI symptom score of ≤4 and ≥5 respectively (4 being the median total modified LENT-SOMA GI symptom score at baseline in one of our previous studies [Citation8]).
Experimental protocol
Each patient underwent the following evaluations: (i) modified Late Effects Normal Tissue – Subjective Objective Management Analytic (LENT-SOMA) GI symptom scales, (ii) generic and disease specific European Organization for Research and Treatment of Cancer (EORTC) HRQoL outcome measures, (iii) anorectal and pudendal nerve function and (iv) anal sphincter morphology.
Modified LENT-SOMA GI symptoms
GI symptoms including stool frequency and consistency, rectal pain, mucous discharge, urgency of defecation and rectal bleeding were evaluated using modified LENT-SOMA scales [Citation8]. Each LENT-SOMA GI symptom was scored on a five point (0–4) categorical scale (1–4 corresponding to the four LENT-SOMA grades, 0 in the modified scale representing the absence of the abnormal symptom) [Citation8].
Generic and disease specific HRQoL measures
Validated EORTC QLQ-C30 and EORTC QLQ-PR25 questionnaires were used to evaluate generic and disease specific HRQoL, respectively [Citation12,Citation13].
The QLQ-C30 HRQoL questionnaires consist of five functional scales, three general symptom scales, a global health quality of life scale and six single items [Citation12].
The disease specific (prostate cancer) module, QLQ-PR25 HRQoL developed for use with QLQ-C30 HRQoL consists of 25 items in four subscales to evaluate bowel symptoms (four items), urinary symptoms (nine items), sexual function (six items) and hormone treatment-related symptoms (six items) [Citation13].
Anorectal function
Urgency of defecation and FI were evaluated by a four point (0–3) categorical scale questionnaire before measurement of anorectal function [Citation14]. Evaluation of each symptom was based on its frequency and severity. A score of 0 represented <1 episode/week, while a score of 3 represented ≥1 episode/day. For severity, 0 denoted absence of the symptom with numerical values up to 3 reflected increasing severity of each symptom [Citation14]. The maximum score for urgency of defecation = 6 and FI = 12, diurnal and nocturnal FI being scored separately [Citation14].
Anorectal motor function was evaluated with a perfused sleeve and multiport anorectal assembly incorporating a highly compliant polyethylene bag located in the rectum. Anorectal pressures were recorded under resting conditions and in response to squeeze and increased intra-abdominal pressure [Citation8,Citation10].
Anorectal sensory function
The sensation reported by the patient (perception of the stimulus, desire to defecate, or pain) during graded rectal distension of the inflatable polythene bag was recorded [Citation8,Citation10].
Pudendal nerve terminal motor latency (PNTML)
PNTML assessment was performed by an accredited technical officer employing a disposable glove-mounted St Mark’s electrode and transrectal nerve stimulation technique (13L40 St Mark’s Pudendal ElectrodeTM, Medtronic Functional Diagnostics A/S, Skovlunde, Denmark). Square wave stimuli of 0.05 ms duration and 10 mA were delivered at one-second intervals. The electromyography (EMG) recordings were acquired via a MacLab or PowerLab 8S or 4S system with a MacLab or PowerLab bioamplifier using ScopeTM (V3.4.3-4.1) software (ADInstruments Pty. Ltd, Castle Hill, NSW, Australia). Multiple recordings were taken on each side and the most well defined action potentials selected for the determination of the latency. The mean latency from these multiple curves was determined. Latency was defined as the time between the stimulation of the pudendal nerve at the level of the ischial spine (the electrical pulse being recorded as a stimulus artifact) and commencement of depolarization of the anal sphincter [Citation15]. A lack of pudendal nerve response was defined as no sphincter contraction on EMG recording in response to stimulus [Citation11].
Anal sphincter morphology
Cross sectional images to determine the maximal thickness of both the IAS and EAS were acquired with a 7.5 MHz rotating ultrasonographic scanner (Bruel and Kjaer, Naerum, Denmark) [Citation8,Citation10].
Derivation of radiation dose volume parameters
Radiation dose volume parameters in the study were based on available CT planning scan data files of 18 patients as some of the archived 3D-CRT data files could not be retrieved.
Organs at risk such as the rectum and anal canal were contoured as solid organs, the latter from the anal verge to the anorectal junction just below the pubo-rectalis sling around the posterior wall of the rectum [Citation11]. The pudendal nerve from its entry in the pelvis through the sciatic notch and its exit in the perineum through the ischial spine was also contoured from the CT planning scans [Citation11].
As only two of the total study population of 25 patients had HDR brachytherapy as a supplement to EBRT, the contribution of HDR brachytherapy to the EBRT derived radiation dose volume parameters of the OARs was not included. Furthermore, it is likely that the contribution of HDR brachytherapy to the EBRT derived dose volume parameters of the OARs in this study is likely to be less than that previously reported [Citation16] because unlike the previous report, the supplementary HDR brachytherapy in both patients in this study was delivered with the transrectal ultrasound probe inserted in the rectum.
Data analysis
Modified LENT-SOMA GI symptoms
The medians of the individual and total GI symptom scores in the whole patient group and the symptomatic and asymptomatic patient sub-groups were calculated.
Generic and disease specific HRQoL measures
For the whole patient group and the patient sub-groups, the median scores of QLQ-C30 scales were derived after transforming the means of the raw scores in individual patients to lie between 0 and 100 [Citation12,Citation17]. For the five functioning scales and one global QoL scale, a higher score denotes a better level of functioning while higher scores in the general symptom and single item scales represent increasing detrimental effects.
The median scores for the four symptom sub-scales of the QLQ-PR25 for the whole patient group and symptomatic and asymptomatic patient sub-groups were derived after transforming the means of the raw scores in individual patients to lie between 0 and 100 [Citation13,Citation17]. As for the QLQ-C30 general symptom and single item scales, higher scores for the four symptom sub-scales of the QLQ-PR25 represent increasing detrimental effects.
Anorectal function
Scores of urgency of defecation and FI were derived for each patient [Citation14]. Semi-automated analysis of the sleeve sensor data determined (i) minimum basal anal pressures and responses to (ii) anal squeeze and (iii) increased intra-abdominal pressure [Citation8,Citation10,Citation14].
The volumes of rectal distension associated with sensory perception and desire to defecate were recorded [Citation8,Citation10,Citation14].
Rectal compliance, based on the maximal slope between 40 and 100 mL of the pressure volume relationship for rectal balloon distension were calculated [Citation8,Citation10,Citation14].
Pudendal nerve terminal motor latency
The percentage of patients who had absent or delayed (>2.6 ms [Citation18]) pudendal nerve response unilaterally and/or bilaterally was calculated.
Anal sphincter morphology
The maximal and mean thickness of both the IAS and EAS were calculated from the endoanal measurements around the anal canal [Citation8,Citation10,Citation14].
Radiation dose volume data
The median and range of the minimum (Dmin), the maximum (Dmax) and the mean (Dmean) doses of the rectum, anal canal and pudendal nerve and the % of each OAR receiving ≥40% (V 40 Gy) and ≥60% (V 60 Gy) of the prescribed EBRT doses to the prostate were derived from available CT planning data of 18 patients [Citation11].
Statistical analysis
The modified LENT-SOMA GI symptom, the generic and disease specific HRQoL, anorectal function and anal sphincteric morphology data were compared between the whole patient group and the data of age matched patients before 3D-CRT for localized prostate carcinoma [Citation19] using the Mann–Whitney U and unpaired t tests. Mann–Whitney’s U test and the unpaired t tests were also used to compare the data between the symptomatic versus asymptomatic sub-groups.
Linear regression was used to analyze the relationships among the modified LENT-SOMA GI symptom and anorectal function and anal morphology parameters in the whole patient group.
χ2 test was used to examine differences between the symptomatic and asymptomatic patients in (i) radiation dose volume parameters and (ii) percentages of patients with absent or delayed pudendal nerve responses unilaterally and/or bilaterally.
A p value of ≤.05 was considered statistically significant in all analyses.
Results
Demographics
The median age (range) of the 25 patients was 77 (64–83) years and median (range) time since completion of EBRT ± HDR brachytherapy was 63 (26–156) months. The median (range) maximum irradiated volume which included the prostate as its target was 203.5 (122.8–389.8) cm3. The median (range) of mean (Dmean) and maximum (Dmax) EBRT doses to the planning target volume (PTV) of the prostate were 70.1 (47.7–74.9) Gy and 71.3 (48.7–76.2) Gy, respectively ().
Comparison between patient groups
There were no differences including age [median (range) = 73 (57–84) years], body weight and body mass index between the age matched and patients in this study.
Comparison between patient sub-groups
There were also no differences between the ages of the symptomatic [median (range) = 77 (69–83) years] versus asymptomatic [median (range) = 75 (64–83) years] patients in this study. The two patients who received HDR brachytherapy supplementary to EBRT were classified as symptomatic and symptomatic patients had completed radiotherapy significantly earlier [median (range) = 52 (25–108) months] than asymptomatic patients [83 (39–156) months], p < .05 (). The median (range) of irradiated prostate volume 198.6 (122.8–252.4), Dmean 70.5 (47.6–74.9) Gy and of Dmax 71.3 (48.7–76.2) Gy were no different in symptomatic (EBRT component only) from the corresponding data of 208.3 (140.2–389.8) mL, 70.3 (69.2–74.6) Gy and 71.3 (69.2–76.0) Gy in asymptomatic patients ().
Modified LENT-SOMA GI symptoms
Comparison between patient groups
Patients in this study had significantly higher modified LENT-SOMA frequency and urgency of defecation, rectal bleeding and mucous discharge scores relative to the data in age matched patients before radiotherapy (Supplementary Table).
Comparison between patient sub-groups
Symptomatic patients had significantly higher modified LENT-SOMA urgency of defecation and rectal bleeding scores compared with asymptomatic patients ().
Table 2. Comparison of modified LENT-SOMA GI symptoms and EORTC generic (QOL-C30) and disease specific (QLQ-PR25) HRQoL data between symptomatic and asymptomatic patients.
Generic and disease specific EORTC HRQoL measures
Comparison between patient groups
Except for lower cognitive functioning scores in the whole patient group, there were no significant differences in the EORTC QLQ-C30 measures in the comparisons with the age matched patients (Supplementary Table). Patients in this study also had worse (higher) bowel symptom scores for the EORTC QLQ-PR25 instrument compared with the data of age matched patients (Supplementary Table).
Comparison between patient sub-groups
Emotional and social functioning scales, diarrhea and fatigue scores as well as global health quality of life measured by the QLQ-C30 instrument were worse in the symptomatic versus the asymptomatic patients (). Urinary and bowel function scores measured by the QLQ-PR25 questionnaires were also worse (higher) for the symptomatic patients ().
Anorectal function
Comparison between patient groups
All parameters of anorectal motor and sensory function except for threshold volumes for sensory perception were significantly worse in the patients in this study compared to baseline data of the aged matched group ().
Table 3. Comparison of anorectal function and anal sphincter morphology data between whole patient group and age matched patients before radiotherapy.
Comparison between patient sub-groups
Fecal incontinence scores were worse in the symptomatic compared to the asymptomatic patients ().
Table 4. Comparison of anorectal function and anal sphincter morphology data between symptomatic and asymptomatic patients.
Pudendal nerve terminal motor latency
Comparison between patient groups
Unilateral and/or bilateral pudendal nerve responses were delayed in 13/24 (54%) of the patients in this study compared to only 2/20 (10%) patients in the aged matched patient group (p < .0001, data not shown).
Comparison between patient sub-groups
Unilateral and/or bilateral pudendal nerve responses were delayed in 9/13 (69%) of symptomatic compared to only 4/11 (36%) of asymptomatic patients (p < .0001, data not shown).
Anal sphincter morphology
Comparison between patient groups
The thickness of both IAS and EAS was less in the patients in this study compared with that of the age matched patients ().
Comparison between patient sub-groups
However, there was no difference in IAS and EAS thickness between symptomatic and asymptomatic patients ().
Linear regression analysis
In the whole patient group, modified LENT-SOMA urgency of defecation and mucous discharge scores were both inversely related to basal anal pressures (r = −0.40, p < .05 and r = −0.39, p < .05 respectively, data not shown).
There were also inverse relationships between LENT-SOMA urgency of defecation scores and anal pressures in response to increased intra-abdominal pressure (r = −0.48, p < .05, data not shown) and between LENT-SOMA mucous discharge scores and anal squeeze pressures (r = −0.52, p < .01, data not shown).
In addition, FI scores in the anorectal function symptom questionnaire (which was directly related to LENT-SOMA mucous discharge scores [r = 0.60, p < .01], data not shown) was inversely related to basal anal (r = −0.39, p = .05, data not shown) and squeeze (r = −0.39, p = .05, data not shown) pressures.
Radiation dose volume parameters
Radiation dose volume data of OARs for the whole patient group in this study are shown in .
Table 5. Median (range) of baseline (planning) dose volume histogram parameters of outer rectal wall, anal wall and pudendal nerve.
Comparisons of the data between symptomatic and asymptomatic patients revealed no significant differences.
The prevalence of delayed unilateral and/or bilateral pudendal nerve conduction was higher for (i) rectal and anal V 40 Gy >65% versus rectal and anal V 40 Gy <65% (p < .0001 for both, data not shown), (ii) rectal and anal Dmax >60 Gy versus Dmax < 60 Gy (p < .0001 for both, data not shown), (iii) pudendal nerve Dmax >60 Gy versus Dmax < 60 Gy (p < .0001, data not shown) and (iv) Anal V 60 Gy >40% versus anal V 60 Gy < 40% (p < .001, data not shown).
Discussion
The study data show that, ≥2 years after 3D-CRT ± HDR brachytherapy for carcinoma of the prostate, patients had (i) impairment of HRQoL bowel symptoms, (ii) worse anorectal motor and sensory function, (iii) thinner internal and EASs and (iv) ∼ 5× greater prevalence of pudendal nerve dysfunction compared to age matched patients before radiotherapy. Furthermore, symptomatic patients had (i) impairment of several generic HRQoL functioning, diarrhea and fatigue measures leading to a reduction in global quality of life scores in addition to worse HRQoL bowel and urinary symptom scores, (ii) worse rectal bleeding, urgency and FI scores and (iii) ∼ 2× higher prevalence of pudendal nerve dysfunction compared to asymptomatic patients after radiotherapy.
The association of impairment of HRQoL bowel symptoms with worse anorectal motor function ≥2 years following 3D-CRT ± HDR brachytherapy in the whole patient group in this study supports previous reports that late anorectal dysfunction rather than rectal bleeding has a greater and more persistent adverse impact on patient reported outcomes [Citation6,Citation8,Citation12]. That symptomatic patients had impairment of a number of generic HRQoL measures including global quality of life and worse bowel symptom scores compared with asymptomatic patients is consistent with a previous report that worse global quality of life was associated with increased FI scores levels after radiotherapy for carcinoma of the prostate [Citation12].
However, the major new observation in the current study of 5× higher prevalence of pudendal nerve dysfunction ≥2 years after 3D-CRT ± HDR brachytherapy compared with age matched patients before radiotherapy has important implications for optimizing current radiotherapy treatment planning and delivery techniques to reduce its prevalence and consequent impairment of anorectal function and HRQoL. Currently, universally recognized OAR in planning radiotherapy for carcinoma of the prostate is the anorectum (combining anal canal and rectum), small and large bowel (only if the pelvic nodes are the target of irradiation) and the bladder although we and others have suggested that the anal canal and rectum should be contoured separately [Citation3,Citation20]. Individual pelvic floor muscles have also been proposed as ‘organs’ at risk [Citation21], The findings of a 5× higher prevalence of pudendal nerve dysfunction 2 years or more after EBRT ± HDR brachytherapy compared with age matched patients before radiotherapy is consistent with the findings of our previous study [Citation11] although no absent pudendal nerve responses were observed in the present study. The lesser impairment of pudendal nerve function in this study is reflected in the absence of evidence of atrophy of the anal sphincters on ultrasound although the internal and EASs were thinner in the patients ≥2 years after 3D-CRT ± HDR brachytherapy compared with age matched patients before radiotherapy. The differences in % of patients treated with 3D-CRT in this study (100%) compared with our previous study (18%) and in the median time since completion of radiotherapy (5 years for present versus 8 years for previous study) suggest that greater sparing by the universal application of 3D-CRT in this study and/or progressive damage to the pudendal nerves in the previous study [Citation20] are likely explanations for the less severe pudendal nerve dysfunction reported here.
The prevalence of unilateral and/or bilateral pudendal nerve injury in the current study was increased if a number of actual (rather than modeled in our previous study [Citation11]) radiation dose volume parameters were exceeded including pudendal nerve, rectal and anal Dmax >60 Gy. Although these parameters have not been previously reported as dose constraints for radiotherapy treatment planning, Dmean anal and V 40 Gy anal have been associated respectively with an increased risk of FI and increased total GI symptom scores after radiotherapy for prostate carcinoma [Citation20,Citation22]. Differences in the definition of the anal canal (contoured separately from the rectum) and statistical analyses of the data between the studies were proposed to account for the finding of a volume [Citation20] as opposed to a dose [Citation22] constraint in the previous studies [Citation20,Citation22]. It is interesting to note that the current study which used the same contouring definitions of rectum and anal canal as one of our previous studies [Citation20] had similar dose volume parameters of the contoured OAR’s and V 40 Gy anal ≥65% was also found to be associated with an increased prevalence of GI OAR damage after radiotherapy.
The data in this study provide further support for separate contouring of the rectum and anal canal in radiotherapy treatment planning for prostate carcinoma and also suggest that the pudendal nerve should be considered as an ‘organ’ at risk of radiation damage. We propose that the maximum dose to each contoured pudendal nerve should not exceed 60 Gy and the V 40 Gy for rectum and anal canal should be kept below 65%. These dose constraints should not be difficult to meet with more sophisticated techniques of radiation dose delivery involving intensity modulation of the radiation treatment (IMRT) beams and could reduce the continuing high (up to 65%) rates of chronic anorectal toxicity reported for IMRT of carcinoma of the prostate [Citation3].
There are several potential weaknesses in this study. These include the study of a relatively small sample of patients retrospectively which hampers meaningful sub-group analyses based on the five previously described late radiation-induced bowel syndromes [Citation23] and the derivation of the radiation dose volume parameters of the OARs based on a single planning CT scan of the pelvis previously reported to be problematic in the proper understanding of dose volume relationships of OARs at risk of late GI toxicity [Citation24]. However, we believe that these are outweighed by the strengths which include (i) a standard treatment technique of the patients and follow-up protocol by a single tertiary institution based radiation oncologist, (ii) comparisons of the data with a population of age matched patients before radiotherapy for carcinoma of the prostate although comparisons with the baseline data of the same patient group would have been preferable and (iii) meaningful significant relationships between the modified LENT-SOMA urgency of defecation, mucous discharge scores and anorectal motor function parameters which not only confirms previous reports that weakness of the IAS and EAS contribute to anorectal dysfunction [Citation7,Citation8,Citation14] but also suggests that the underlying etiology of the weakness is neurogenic. Nevertheless, the long time delay between development of symptoms and investigation in this and our previous study [Citation11] suggest the need for well designed prospective studies with prolonged follow up to confirm the findings reported in this study.
Eng__Eric__et_al._Supplementary_table.docx
Download MS Word (12.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jani AB, Hellman S. Early prostate cancer: clinical decision-making. Lancet. 2003;361:1045–1053.
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–308.
- Smeenk RJ, The BS, Butler EB, et al. Is there a role for endorectal balloons in prostate radiotherapy? A systematic review. Radiother Oncol. 2010;96:277–282.
- al-Abany M, Helgason AR, Cronqvist AK, et al. Long-term symptoms after external beam radiation therapy for prostate cancer with three or four fields. Acta Oncol. 2002;41:532–542.
- Heemsbergen WD, Hoogeman MS, Hart GA, et al. Gastrointestinal toxicity and its relation to dose distributions in the anorectal region of prostate cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1011–1018.
- Maeda Y, Hoyer M, Lundby L, et al. Faecal incontinence following radiotherapy for prostate cancer: a systematic review. Radiother Oncol. 2011;98:145–153.
- Yeoh E, Tam W, Schoeman M, et al. Argon plasma coagulation therapy versus topical formalin for intractable rectal bleeding and anorectal dysfunction after radiotherapy for prostate carcinoma. Int J Radiat Oncol Biol Phys. 2013;87:954–959.
- Yeoh EK, Holloway RH, Fraser RJ, et al. Pathophysiology and natural history of anorectal sequelae following radiation therapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2012;84:e593–e599.
- Krol R, Hopman WP, Smeenk RJ, et al. Increased rectal wall stiffness after prostate radiotherapy: relation with fecal urgency. Neurogastroenterol Motil. 2012;24:339–e166.
- Yeoh EK, Bartholomeusz DL, Holloway RH, et al. Disturbed colonic motility contributes to anorectal symptoms and dysfunction after radiotherapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2010;78:773–780.
- Loganathan A, Schloithe AC, Hutton J, et al. Pudendal nerve injury in men with fecal incontinence after radiotherapy for prostate cancer. Acta Oncol. 2015;54:882–888.
- Geinitz H, Zimmermann FB, Thamm R, et al. Late rectal symptoms and quality of life after conformal radiotherapy for prostate cancer. Radiother Oncol. 2006;79:341–347.
- Denham JW, Wilcox C, Joseph D, et al. Quality of life in men with locally advanced prostate cancer treated with leuprorelin and radiotherapy with or without zoledronic acid (TROG 03.04 RADAR): secondary endpoints from a randomised phase 3 factorial trial. Lancet Oncol. 2012;13:1260–1270.
- Yeoh EE, Holloway RH, Fraser RJ, et al. Anorectal dysfunction increases with time following radiation therapy for carcinoma of the prostate. Am J Gastroenterol. 2004;99:361–369.
- Bright T, Kapoor R, Voyvodich F, et al. The use of a balloon catheter to improve evaluation in anorectal manometry. Colorectal Dis. 2005;7:47.
- Pettersson N, Johansson K-A, Alsadius D, et al. A method to estimate composite doses for organs at risk in prostate cancer patients treated with EBRT in combination with HDR brachytherapy. Acta Oncol. 2014;53:815–821.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in Oncology. J Natl Cancer Inst. 1993;85:365–376.
- Voyvodic F, Rieger NA, Skinner S, et al. Endosonographic imaging of anal sphincter injury: does the size of the tear correlate with the degree of dysfunction? Dis Colon Rectum. 2003;46:735–741.
- Botten R, DiMAtteo AC, Sharma KG, et al. Endorectal balloon during image guided radiation therapy for carcinoma of the prostate reduces rectal bleeding and sensory dysfunction and improves health related quality of life outcomes at 2 years. Gastroenterology. 2016;150:S937.
- Yeoh EK, Krol R, Dhillon VS, et al. Predictors of radiation-induced morbidity following radiotherapy for carcinoma of the prostate. Acta Oncol. 2016;55:604–610.
- Smeenk RJ, Hoffman AL, Hopman, et al. Dose effect relationships of individual pelvic floor muscles and anorectal complaints after pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:636–644.
- Alsadius D, Hedelin M, Lundstedt D, et al. Mean absorbed dose to the anal-sphincter region and fecal leakage among irradiated prostate cancer survivors. Int J Radiat Oncol Biol Phys. 2012;84:e181–e185.
- Steineck G, Sjoberg F, Skokic V, et al. Late radiation-induced bowel syndromes, tobacco smoking, age at treatment and time since treatment – gynecological cancer survivors. Acta Oncol. 2017;65:682–691.
- Waldenstrom A-C, Alsadius D, Pettersson N, et al. Variations in position and volume of organs at risk in the small pelvis. Acta Oncol. 2010;49:491–499.