Abstract
Introduction: The influence of intra-tumoral heterogeneity on the evaluation of immunohistochemical (IHC) biomarker expression may affect the analytical validity of new biomarkers substantially and hence compromise the clinical utility. The aim of this study was to examine the influence of intra-tumoral heterogeneity as well as inter-observer variability on the evaluation of various IHC markers with potential prognostic impact in breast cancer (BCL2, E-cadherin, EGFR, EMMPRIN and Ki-67).
Material and methods: From each of 27 breast cancer patients, two tumor-containing paraffin blocks were chosen. Intra-tumoral heterogeneity was evaluated (1) within a single tumor-containing paraffin block (‘intra-block agreement’) by comparing information from a central, a peripheral tissue microarray (TMA) core and a whole slide section (WS), (2) between two different tumor-containing blocks from the same primary tumor (‘inter-block agreement’) by comparing information from TMA cores (central/peripheral) and WS. IHC markers on WS and TMA cores were evaluated by two observers independently, and agreements were estimated by Kappa statistics.
Results: For BCL2, E-cadherin and EGFR, an almost perfect intra- and inter-block agreement was found. EMMPRIN and Ki-67 showed a more heterogeneous expression with moderate to substantial intra-block agreements. For both stainings, there was a moderate inter-block agreement that improved slightly for EMMPRIN, when using WS instead of TMA cores. Inter-observer agreements were found to be almost perfect for BCL2, E-cadherin and EGFR (WS: κ > 0.82, TMAs: κ > 0.90), substantial for EMMPRIN (κ > 0.63), but only fair to moderate for Ki-67 (WS: κ = 0.54, TMAs: κ = 0.33).
Conclusions: BCL2, E-cadherin and EGFR were found to be homogeneously expressed, whereas EMMPRIN and Ki-67 showed a more pronounced degree of intra-tumoral heterogeneity. The results emphasize the importance of securing the analytical validity of new biomarkers by examining the intra-tumoral heterogeneity of immunohistochemical stainings applied to TMA cores individually in each type of cancer.
Introduction
Individualizing cancer therapy is the pivot of current cancer research, and large scale gene-expression analyses are carried out in the search for potential prognostic and predictive biomarkers. Immunohistochemical (IHC) stainings, highlighting protein expression, represent an economical and readily available alternative to gene expression analysis, and may potentially aid the integration of new prognostic/predictive markers into the daily clinical practice in an easy and standardized way. Studies encompassing large number of patients are, however, necessary to corroborate the actual relevance of the different markers in order to secure the clinical validity. The tissue microarray (TMA) procedure, examining multiple tiny paraffin-embedded tumor biopsies, facilitates analysis of such large-scaled IHC studies [Citation1] and reduces the expenses. One of the most frequently presented concerns, when using TMAs instead of whole slide sections (WS), is that the small-sized TMA cores may not be representative for the tumor bulk [Citation2–4]. The influence of intra-tumoral heterogeniety on the evaluation of biomarker expression may thus affect the analytical validity of the biomarker and hence the clinical utility. Publications on biomarkers differ, nevertheless, substantially in the use of either WS or TMAs, and when TMAs are used, it is often not well-described, which area of the tumor the TMA core has been sampled from (e.g., invasive front or center of tumor). We have previously shown a fine agreement between central TMA cores and WS for the estrogen receptor and the HER-2 receptor, as well as an acceptable agreement for the progesterone receptor and for carbonic anhydrase 9 after staining just a single central or peripheral TMA core biopsy [Citation5,Citation6].
In this study, we have examined a larger panel of biomarkers with potential prognostic value in breast cancer (BCL2, E-cadherin, EGFR, EMMPRIN, and Ki-67), using both TMAs and WS in order to examine the influence of intra-tumoral heterogenity on the interpretation of these markers. B-cell lymphoma 2 (BCL2) is an anti-apoptosis protein shown to be of independent prognostic value in breast cancer [Citation7]. BCL2 is an estrogen responsive gene and the prognostic role may be subtype specific [Citation8]. Previous studies showing the prognostic value of BCL2 have used either WS [Citation8] or TMAs [Citation9]. E-cadherin is an adhesion molecule, which is absent in the majority of invasive lobular carcinomas of the breast. In invasive ductal carcinomas, loss of E-cadherin may be an independent, negative prognostic indicator [Citation10]. EGFR expression is linked to especially triple negative breast cancer (TNBC)/basal-like tumors, and has been found to be associated with an inferior prognosis [Citation11]. The presence of EGFR overexpression may represent a potential target in TNBC, but the frequency of EGFR protein expression as determined by IHC in TNBC, has been found to vary significantly depending on, e.g., evaluation technique and antibodies used [Citation12]. EMMPRIN (CD147) is a transmembrane glycoprotein of the immunoglobulin superfamily, and overexpression has been found to be associated with an invasive phenotype as well as with metastasis in various types of cancers, including subtypes of breast cancer [Citation13]. The expression has, however, been found to be heterogeneous [Citation14]. The assessment of tumor proliferation through immunhistochemical detection of Ki-67 has been practiced for many years, but the clinical utility of this biomarker and its prognostic value is complicated by the lack of a standardized procedure for assessment [Citation15]. Ki-67 has, nevertheless, become a key player, when discussing breast cancer prognosis.
The aim of this study was to examine the influence of intra-tumoral heterogeneity and inter-observer agreement on the evaluation of various IHC markers with potential prognostic impact in breast cancer (BCL2, E-cadherin, EGFR, EMMPRIN, and Ki-67). Intra-tumoral heterogeneity was evaluated as ‘intra-block agreement’ by comparing the information from a central, a peripheral TMA core and a WS within a single tumor-containing paraffin block, and as ‘inter-block’ agreement by comparing information from TMA cores (central/peripheral) and WS between two different tumor-containing paraffin blocks from the same primary tumor. Finally, inter-observer agreement was calculated for WS and TMA cores, independently evaluated by two observers.
Materials and methods
The study material encompassed tumor-containing, formalin-fixed, paraffin blocks (FFPE) from 27 patients diagnosed with breast cancer in 1992–1993, as described in detail elsewhere [Citation5]. The histological subtypes included invasive ductal carcinomas (N = 22) as well as invasive lobular carcinomas (N = 5). From each patient, two FFPE blocks were sampled, sliced, and stained with hematoxylin and eosin. Invasive carcinoma in the block was verified and marked, and from the invasive areas one 1 mm core was taken from the tumors central and peripheral compartments, respectively, and transferred to recipient TMA blocks. Four micrometer thick sections were cut from the TMA recipient blocks and the original donor blocks (supplementary Figure 1). Sections were immunohistochemically stained for BCL2, E-cadherin, EGFR, EMMPRIN and Ki-67 (supplementary Table 1 includes information on antibodies and staining procedures). Sections from other breast tumors, previously defined as positive, when stained with the respective antibodies, were additionally stained with and without the primary antibody and served as positive and negative controls.
For the individual stainings, percentage of invasive tumor with cytoplasmic BCL2 staining, membranous E-cadherin, EGFR and EMMPRIN, and nuclear Ki-67 were recorded, as was intensity (on a scale from 0 to 3). All sections were scored semi-quantitatively by two observers (MK and TT or MK and FBS). Afterwards, scores from all six stainings were dichotomized into positive and negative. The cut off used for distinguishing between negative and positive samples was 10% (with any intensity) for BCL2, E-cadherin, EGFR, and EMMPRIN, according to previous publications [Citation8,Citation16–18]. An optimal cut off point for Ki-67 has been widely discussed, but in this study a tumor was defined as positive (‘high expression’), if at least 20% of invasive tumor nuclei stained with any intensity according to the St. Gallen consensus 2013 [Citation19]. The percentages were recorded semi-quantitatively as an average of invasive tumor cells with positive reaction in comparison to invasive tumor cells in total.
Statistics
Inter-observer agreements and intra-observer agreements within as well as between tumor containing blocks and TMA cores are expressed as Kappa coefficients. Kappa statistics is explained as the chance-corrected proportional agreement, and possible values range from +1 (perfect agreement) via 0 (no agreement above that expected by chance) to –1 (complete disagreement). A rough interpretation of Kappa values rates < +0.2 as poor, +0.2 to +0.4 as fair, +0.4 to +0.6 as moderate, +0.6 to +0.8 as substantial, and +0.8 to +1.0 as almost perfect agreement [Citation20]. Calculations were performed using Stata 15.0.
Results
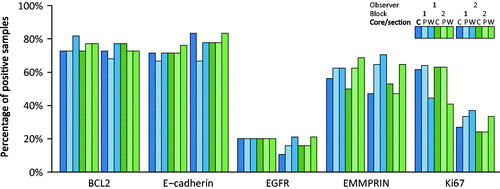
The frequency of positive tumors varied for the different markers (). On average, including all three cores and sections for both blocks and for both observers for 27 patients (N = 324), 223 out of 306 (73%) of the samples were positive for BCL2. For the other markers, the frequencies of positive tumors were E-cadherin: 73% (219/301), EGFR 19% (58/302), EMMPRIN: 58% (165/286), and Ki67: 43% (133/311).
Figure 1. Frequency of positive tumors for markers BCL2, E-cadherin, EGFR, EMMPRIN, and Ki-67. Immunohistochemical analyzes of markers were interpreted from TMA cores, one central (C) and one peripheral (P), and whole slide sections (W) each from two blocks (1 and 2) from each tumor. All cases have been scored by two observers, 1 and 2, respectively. Only tumors that were interpretable on all cores and sections were included in the analysis.
To evaluate intra- and inter-block agreements as well as inter-observer agreements, Kappa values were calculated for various comparisons as listed in . Comparisons were mainly performed between individual cores and sections. In some cases, a cumulated score was used for the TMA cores of a single block, scoring the sample as positive if either or both the central or the peripheral core was positive (listed as ‘central + peripheral’ in ).
Table 1. Kappa values describing comparisons between immunohistochemial stainings for BCL2, E-Cadherin, EGFR, EMMPRIN (10% cut offs), and Ki67 (20% cut offs) of 108 TMA biopsies and 54 whole slide sections (WS) from 27 breast carcinomas.
In general, BCL2, E-cadherin and EGFR were found to be homogeneously expressed, and showed little intra-tumoral variation within and between blocks. The Kappa values for comparison between central and peripheral TMA cores within a single block exceeded 0.81 corresponding to almost perfect agreement, and agreement between cores (central or peripheral) and WS were very high for E-cadherin and substantial to almost perfect for BCL2 and EGFR. The agreements for the three stainings did not increase further, when cumulating the central and the peripheral TMA core and comparing to the corresponding WS from the same tumor-containing paraffin block. For BCL2 and E-cadherin, there was in general a very high inter-block agreement, using either TMA cores or WS, whereas the agreement between information from WS from two different blocks were slightly lower but still substantial for EGFR. A very high inter-observer agreement was further found for BCL2, E-cadherin and EGFR, using either TMAs or WS, with Kappa values in the almost perfect range.
For EMMPRIN, intra-tumoral heterogeneity did, however, affect the expression on IHC with considerable intra- as well as inter-block variation. indicates that the expression of EMMPRIN was higher in cores taken from the periphery of the tumor as compared to cores from the center, and the Kappa values for comparison between the central and the peripheral TMA cores within the blocks were in the moderate range. further indicates that a higher percentage of positive cases were found, when evaluating WS as compared to TMA cores. Agreement between cores (central or peripheral) and corresponding WS were, however, found to be substantial for observer 1, but only moderate for observer 2. When cumulating the central and the peripheral TMA core and comparing to a WS, the Kappa values increased slightly, but were still within the moderate to substantial range. The inter-block agreement for EMMPRIN, using either cores or WS, was found to be highly variable for the two observers with Kappa values ranging from fair to almost perfect and with higher Kappa values found for observer 1 than for observer 2. Despite the intra-tumoral heterogeneity, a substantial inter-observer agreement was found for EMMPRIN for both TMA cores and WS.
The intra-observer agreements for Ki-67 was substantial to almost perfect, when comparing central and peripheral TMA cores, and moderate to substantial when comparing cores (central or peripheral) and corresponding WS. The agreements did not increase, when cumulating the central and the peripheral TMA core and comparing to a WS. As for EMMPRIN, the inter-block agreements were highly variable for the two observers, when evaluating TMA cores, with Kappa values ranging from fair to almost perfect. For both observers, a substantial agreement was, however, found between WS from two different blocks with Kappa values exceeding 0.76. shows a difference in individual scaling for Ki-67 with a substantially higher number of positive cases, especially on TMA cores, for observer 1 as compared to observer 2. The inter-observer agreement for Ki67 was, accordingly, only fair, when using TMA cores, but increased to moderate, when using WS.
Discussion
The results of the present study show BCL2, E-cadherin and EGFR to be homogeneously expressed in breast cancer and indicate that TMA cores are representative for the tumor bulk for these three biomarkers. The findings are in line with previous findings regarding these markers in breast cancer [Citation21,Citation22]. Nassar et al. [Citation22] examined three cores from different regions of breast carcinomas and normal tissue, and similarly found that E-cadherin and EGFR lacked heterogeneity. The general consensus is that two to four tissue cores are representative with 95–97% concordance rates [Citation23,Citation24]. For BCL2, E-cadherin and EGFR, the present results, however, indicate that it is sufficient to use TMAs constructed with only one 1 mm core to determine the IHC expression in a breast carcinoma. Furthermore, it does not seem to influence the results, whether the TMA core is taken from the center or from the invasive front of the tumor. The results further shows that the variation between different tumor-containing blocks is negligible, and that choosing material from a single representative block is sufficient.
On the contrary, the results for EMMPRIN indicated that intra-tumoral heterogeneity affects the interpretation of the staining. The information from TMA cores were found to be in better concordance with information from the corresponding WS, when combining information from both a central and a peripheral core. It is, therefore, advisable to include more cores, preferably from different areas of the tumor, when constructing TMAs for evaluating EMMPRIN. There was, however, still only moderate agreement between the data obtained from two different blocks, meaning that the intra-tumoral heterogeneity cannot be fully compensated for, if evaluating the expression of EMMPRIN from a WS instead of from TMA cores. We have not found any other studies having validated EMMPRIN staining of TMA cores.
For Ki-67, intra-tumoral heterogeneity was found to affect the interpretation, which is in accordance with the current knowledge that Ki-67 harbors both spatial and temporal heterogeneity [Citation22,Citation25]. Muftah et al. [Citation26] have examined the concordance between a WS and a single peripheral core, and found a low concordance, when dichotomized (κ = 0.30), and a moderate concordance when using Ki-67 as a continuous variable (Intraclass coefficient = 0.61). On the contrary, Batistatou et al. [Citation27] found a very high correlation between TMA cores and WS (κ = 0.95), using a 14% cut off, and only a single core from a non-specified area. In our study, however, the inter-observer variability seemed to be an issue of even more impact.
Previous publications have shown that the analytical validity is especially low in the intermediate range for Ki-67, whereas there may be evidence for a clinical utility of very low and high levels of Ki-67 [Citation28], and estimation of Ki-67 has been found especially difficult to standardize in the intermediate range [Citation25]. In our study, we only evaluated the agreement of dichotomized results on the basis of a 20% cut off. Evaluating agreements in categories of, e.g., very low, intermediate, and high may have shown higher agreements in the very low and very high categories. Actually, it may be that the observed poor inter-observer agreements are primarily due to variations in the intermediate range. It was, however, not within the scope of this study to test the agreement within different categories of expression using different cut off points. The present study was carried out before the international recommendations for Ki-67 assessment was published [Citation29], and the expression was not evaluated in the invasive edge only or in hot spots. Counting a specific number of cells, e.g., 500 tumor cells was also not pre-specified, and the subjectively estimated, average percentage from WS and TMA cores were recorded. It is highly likely that the intra- and inter-block agreements would have been higher, if using a different assessment method for Ki-67. Denkert et al. [Citation25] have, assisted by a mathematical model, shown that counting at least 500–1000 cells is necessary to achieve an acceptable error rate, when using a 15% cut off value for Ki-67. The present results did not support that Ki-67 expression was higher in the invasive edge of the tumor than in the center as could have been expected (), and did not indicate that WS instead of TMA cores should be favored.
Intra-tumoral heterogeneity may apply to intrinsic as well as extrinsic factors, meaning that the heterogeneity may arise from subclones in the tumor or may be due to differences in differentiation in the tumor or variations in the tumor-microenvironment. The heterogeneity may, however, also be due to pre-analytical factors related to variation in pre-fixation time or fixation etc. [Citation30]. The extrinsic factors are, nevertheless, not likely to have contributed much to the inter-block variability in this study, since the two blocks were processed from the same tumor and as such handled under the same conditions.
It has been anticipated that the TMA technology would become an important vehicle in defining predictive biomarkers for future biospecific therapies [Citation31]. Ilyas et al. [Citation30] provided guidelines for conducting experiments with TMAs and suggested that the guidelines should be used as a supplement to the REMARK criteria for reporting IHC studies [Citation32]. This study supports the findings by Ilyas et al. [Citation30], and emphasizes the importance of examining the intra-tumoral heterogeneity of IHC stainings applied to TMA cores individually in each type of cancer as part of securing the analytical validity of new biomarkers.
Trine_Tramm_et_al._Supplementary_material.pdf
Download PDF (230.7 KB)Acknowledgements
The authors thank Birthe Hermansen and Mogens J. Johannsen for excellent technical assistance.
Disclosure statement
TT has received royalties from Pfizer and Roche A/S for lectures given. The funding sources had no role in the manuscript production. No potential conflicts of interest were disclosed by the other authors.
Additional information
Funding
References
- Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847.
- Bubendorf L, Nocito A, Moch H, et al. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001;195:72–79.
- Horvath L, Henshall S. The application of tissue microarrays to cancer research. Pathology. 2001;33:125–129.
- Simon R, Sauter G. Tissue microarrays for miniaturized high-throughput molecular profiling of tumors. Exp Hematol. 2002;30:1365–1372.
- Kyndi M, Sorensen FB, Knudsen H, et al. Tissue microarrays compared with whole sections and biochemical analyses. A subgroup analysis of DBCG 82 b&c. Acta Oncol. 2008;47:591–599.
- Kyndi M, Sorensen FB, Knudsen H, et al. Carbonic anhydrase IX and response to postmastectomy radiotherapy in high-risk breast cancer: a subgroup analysis of the DBCG82 b and c trials. Breast Cancer Res. 2008;10:R24.
- Callagy GM, Webber MJ, Pharoah PD, et al. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008;8:153
- Eom YH, Kim HS, Lee A, et al. BCL2 as a subtype-specific prognostic marker for breast cancer. J Breast Cancer. 2016;19:252–260.
- Callagy GM, Pharoah PD, Pinder SE, et al. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res. 2006;12:2468–2475.
- Gould Rothberg BE, Bracken MB. E-cadherin immunohistochemical expression as a prognostic factor in infiltrating ductal carcinoma of the breast: a systematic review and meta-analysis. Breast Cancer Res Treat. 2006;100:139–148.
- Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374.
- Nakai K, Hung MC, Yamaguchi H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am J Cancer Res. 2016;6:1609–1623.
- Zhao S, Ma W, Zhang M, et al. High expression of CD147 and MMP-9 is correlated with poor prognosis of triple-negative breast cancer (TNBC) patients. Med Oncol. 2013;30:335–012-0335-4.
- Grass GD, Tolliver LB, Bratoeva M, et al. CD147, CD44, and the epidermal growth factor receptor (EGFR) signaling pathway cooperate to regulate breast epithelial cell invasiveness. J Biol Chem. 2013;288:26089–26104.
- Pathmanathan N, Balleine RL. Ki67 and proliferation in breast cancer. J Clin Pathol. 2013;66:512–516.
- Liu Y, Xin T, Jiang QY, et al. CD147, MMP9 expression and clinical significance of basal-like breast cancer. Med Oncol. 2013;30:366.
- Howat WJ, Blows FM, Provenzano E, et al. Performance of automated scoring of ER, PR, HER2, CK5/6 and EGFR in breast cancer tissue microarrays in the Breast Cancer Association Consortium. J Pathol Clin Res. 2014;1:18–32.
- Meijnen P, Peterse JL, Antonini N, et al. Immunohistochemical categorisation of ductal carcinoma in situ of the breast. Br J Cancer. 2008;98:137–142.
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174.
- Chhieng DC, Frost AR, Niwas S, et al. Intratumor heterogeneity of biomarker expression in breast carcinomas. Biotech Histochem. 2004;79:25–36.
- Nassar A, Radhakrishnan A, Cabrero IA, et al. Intratumoral heterogeneity of immunohistochemical marker expression in breast carcinoma: a tissue microarray-based study. Appl Immunohistochem Mol Morphol. 2010;18:433–441.
- Simon R, Mirlacher M, Sauter G. Tissue microarrays. BioTechniques. 2004;36:98–105.
- Watanabe A, Cornelison R, Hostetter G. Tissue microarrays: applications in genomic research. Expert Rev Mol Diagn. 2005;5:171–181.
- Denkert C, Budczies J, von Minckwitz G, et al. Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast. 2015;24 Suppl 2:S67–S72.
- Muftah AA, Aleskandarany MA, Al-Kaabi MM, et al. Ki67 expression in invasive breast cancer: the use of tissue microarrays compared with whole tissue sections. Breast Cancer Res Treat. 2017;164:341–348.
- Batistatou A, Televantou D, Bobos M, et al. Evaluation of current prognostic and predictive markers in breast cancer: a validation study of tissue microarrays. Anticancer Res. 2013;33:2139–2145.
- Varga Z, Diebold J, Dommann-Scherrer C, et al. How reliable is Ki-67 immunohistochemistry in grade 2 breast carcinomas? A QA study of the Swiss Working Group of Breast- and Gynecopathologists. PLoS One. 2012;7:e37379.
- Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664.
- Ilyas M, Grabsch H, Ellis IO, et al. Guidelines and considerations for conducting experiments using tissue microarrays. Histopathology. 2013;62:827–839.
- Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J Clin Oncol. 2008;26:5630–5637.
- Altman DG, McShane LM, Sauerbrei W, et al. Reporting Recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012;9:e1001216.
