Introduction
Management of head and neck cancer is organized in a fairly unified and centralized manner in the five Northern European countries with a population of 27 M people [Citation1]. However, there are no consensus-based site-specific treatment protocols for these tumours in this region although national guidelines exist. Furthermore, guidelines should be created for rare cancers to enable prospective collection of data from multicentre studies.
Nasopharyngeal cancer (NPC) is a rather uncommon malignancy in the Western world. NPC incidence varies greatly between continents, and disparities exist even among specific Asian-American populations [Citation2]. The Nordic countries, that is, Denmark, Finland, Iceland, Norway and Sweden, remain a low-incidence region with age-standardized incidence rates of 0.2 and 0.3/100,000 for females and males, respectively, during 2010–2014 [Citation3].
NPC is highly sensitive to both radiotherapy (RT) and chemotherapy (CT). Thus, concurrent chemoradiotherapy (CRT) remains the standard treatment for loco-regionally advanced NPC, that is, conventional fractionated RT with concurrent cisplatin and 5-FU, with or without adjuvant CT. The role of adjuvant cisplatin and fluorouracil (PF) following CRT remains debatable [Citation4]. Results from a recent meta-analysis confirm that the addition of concomitant CT to RT significantly improves survival in this patient population [Citation5]. However, there are preliminary results also of changing to an induction-concurrent sequence, but the benefit of this protocol still remains uncertain [Citation6]. Further, intensity-modulated RT (IMRT) has been reported to provide an improved loco-regional recurrence-free survival in both early and advanced loco-regional NPC compared with 2D-CRT [Citation7]. Also, in dosimetric comparisons, intensity-modulated proton therapy (IMPT) seems to have the potential to reduce dose to organs at risk when compared with IMRT and with excellent loco-regional control rates [Citation8]. Randomized prospective trials directly comparing these two RT techniques are, however, lacking.
Early results of robotic nasopharyngectomy have shown a high local control rate. Further, operating time seems to be comparable to open surgery and postoperative morbidity rates remain low [Citation9]. Currently, in some regions, quantification of plasma EBV DNA is routinely used for monitoring patients with NPC and predicting outcome of treatment [Citation10]. The prognosis of NPC has improved significantly over the past three decades because of advances in various aspects of disease management as indicated above [Citation4].
No previous report has evaluated the current treatment protocols and outcome of NPC in Northern Europe. The aim of this Nordic collaborative study was to obtain population-based data on the current management strategies and outcome of NPC in the five Nordic countries.
Patients and methods
Patients
This study consists of all patients with a NPC diagnosed and treated between 1 January 2005 and 31 December 2009 at the 23 Nordic centres located in Denmark, Finland, Iceland, Norway and Sweden managing these patients. The hospital records were reviewed to collect details about age, sex, tumour histology (WHO criteria), TNM classification, stage, intent of treatment, details of treatment (radiotherapy [RT], chemotherapy [CT] and chemoradiotherapy [CRT], neck dissection [ND]), tumour recurrence and tumour and vital status at the last follow-up.
The study cohort consists of 358 consecutive patients. Complete medical records from 344 (96.1%) patients were available for the analysis. The median age was 57 years (range: 15–98), and 241 (70.1%) of the 344 patients were male. Tumor WHO criteria for the histological grade of differentiation were as follows: I 11%, II 35% and III 45%. The stage distribution of the cancers was as follows: I 7.5%, II 16.2%, III 33.5%, IVa 27.1%, IVb 7% and IVc 7%. All patients had a minimum follow-up time of three years or until death.
The national and/or institutional authorities approved the study protocol in each participating country.
Statistical analysis
Survival was analysed with the Kaplan–Meier product-limit method. Overall survival was calculated from the date of the diagnosis of NPC to the date of death from any cause, censoring the patients who were alive on the date of the last contact. Disease-specific survival was calculated from the date of the diagnosis to death considered to result from NPC, censoring the patients who were alive and those who had died from a competing cause on the date of death. Loco-regional survival was calculated from the date of the diagnosis to the date of cancer recurrence in the locoregional region or to death, censoring the patients who were alive without locoregional recurrence.
Results
Eighty-eight percent of the 344 patients were treated with curative intent. The used RT technique was 3D-CRT for 181 (52.6%) and IMRT for 163 (47.4%) patients. In IMRT, the simultaneous integrated boost (SIB) technique was used for 21 patients. Seventeen (5%) of the 344 patients received a brachytherapy boost after RT. Proton therapy was given as the primary treatment in two cases: one patient received definitive proton therapy and one had RT boosted with protons.
A high (≥70 Gy), intermediate (65–69 Gy) and low cumulative dose (<65 Gy) of RT was given to 107 (31.2%), 183 (53.2%) and 43 (12.5%) patients, respectively (data missing for 11 patients). Seventeen (5%) patients had hyperfractionated treatment. Concurrent CT was given to 228 (66.3%) out of the 344 patients. Seventy-two (20.9%) patients received CRT with cisplatin 100 mg/m2 every third week, and 154 (44.8%) patients with cisplatin 40 mg/m2 once a week. Nimorazole combined with accelerated RT was administered to five patients at one centre.
Neoadjuvant CT was given to 82 (23.8%) and adjuvant CT to 26 (7.6%) of the 344 patients. In most patients, adjuvant CT consisted of three cycles of cisplatin and fluorouracil. Other combinations used were carboplatin-gemcitabine (n = 7), cisplatin plus docetaxel (n = 4), docetaxel (n = 2) and paclitaxel for one patient. Neck dissection was performed primarily in 16 (4.7%) cases, and as salvage surgery in 18 (5.2%) cases.
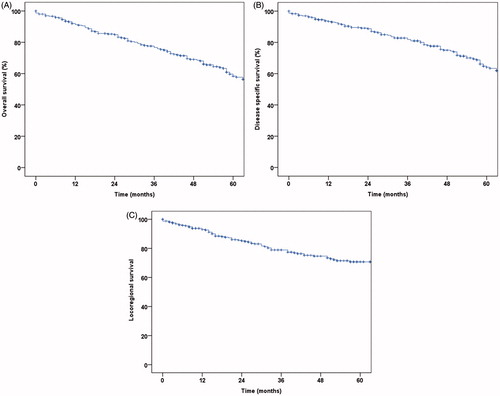
Fifty-five (16%) patients had a local recurrence, 34 (9.9%) neck recurrence and 65 (18.9%) distant recurrence. At the end of follow-up, 172 (50%) patients had no evidence of disease, 13 (3.8%) were alive with disease, 115 (33.4%) had died of disease and 39 (11.3%) of other causes. The 3-year and 5-year survival estimates, respectively, were 77.9% (SE ±2.6) and 60.2% (SE ±3.4) for overall survival, 82.8% (SE ±2.4) and 66.4% (SE ±3.4) for disease-specific survival, and 78.9% (SE ±2.6) and 70.7% (SE ±3.1) for loco-regional tumour control ().
Discussion
Northern Europe, that is, the five Nordic countries forms a culturally homogenous region with a population of 27 M people and with similar organizational setting for health care. However, this region still has no unified treatment protocol for the management of head and neck cancer [Citation1]. The Scandinavian Society for Head and Neck Oncology (www.sshno.org) has the aim to harmonize the current guidelines for various head and neck cancers in this region, and NPC as a rare cancer type was chosen as one of the sites to be addressed for this purpose. Here, we describe the treatment approach for nasopharyngeal cancer at the 23 university hospitals which are responsible for the management of this disease.
The present survey revealed that the treatment guidelines for NPC do vary between the academic centres in the region. Differences in radiotherapy techniques, concomitant chemotherapy and also in adjuvant chemotherapy were observed. The general prescription of CRT, however, was in accordance with the stage distribution of the study population as 67.6% had locally advanced stage III or IV disease, and 66.2% received CRT.
These numbers are in line with the existing guidelines for the management of NPC. Recent publications highlight IMRT, with or without concurrent chemotherapy, as the mainstay of curative treatment, with concurrent CT for stages III and IV disease [Citation11,Citation12]. In this respect, the results of the present survey warrant further development of the treatment guidelines in Northern Europe, since some variation was observed.
Reports on the outcome for NPC in Northern Europe are rare. The results on survival outcome in the present study may be compared to international data gathered from randomized trials [Citation5]. This comparison shows a slightly lower overall survival rate in our study, but equivalent disease-specific/progression-free survival rates. It needs to be emphasized, though, that the present study was population-based and included all patients with NPC without restrictions, whereas the selection criteria for phase III trials favour patients with good performance status.
Unified treatment guidelines for NPC in the Nordic countries should be made to facilitate improving survival outcomes and to reduce treatment-associated morbidity. The health care organizations in the Nordic countries are rather similar, mainly funded by central governments and run by public hospitals, particularly considering cancer treatment. Additionally, all the Nordic countries have population-based cancer registries. This common structure should facilitate implementation of standard logistics and general treatment strategies, including clinical guidelines for rare cancer diseases such as NPC. It remains obvious that centralization of the treatment of rare cancers should be further pursued. Although management of head and neck cancer, in general, is centralized to the university hospitals in all of these countries, some of the centres manage only one to three NPC cases annually. Due to this small number of NPC patients in the Nordic countries, means to improve the treating team experience should be actively sought.
Conclusions
NPC is a relatively rare type of head and neck cancer in the Nordic countries. Management protocol for this disease varies between centres responsible for its treatment in this area despite a common public health care structure. The results from this survey indicate that the outcome results of NPC in this region compare well with the results available from other regions, but further benefits might be obtained from mutually agreed detailed treatment guidelines.
Disclosure statement
All authors declare no conflict of interest.
Additional information
Funding
References
- Mäkitie A, Cange HH, Hammarstedt-Nordenvall L, et al. Head and neck cancer management in the Nordic countries: an effort to harmonize treatment. Eur Arch Otorhinolaryngol. 2017;274:2363–2365.
- Jin H, Pinheiro PS, Xu J, et al. Cancer incidence among Asian American populations in the United States, 2009–2011. Int J Cancer. 2016;138:2136–2145.
- Engholm G, Ferlay J, Christensen N, et al. NORDCAN: cancer incidence, mortality, prevalence and survival in the Nordic Countries, version 7.3 (08.07.2016). Association of the Nordic cancer registries. Danish Cancer Society. Available from: http://www.ancr.nu
- Lee AW, Ma BB, Ng WT, et al. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33:3356–3364.
- Blanchard P, Lee A, Marguet S, MAC-NPC Collaborative Group, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16:645–655.
- Lee AW, Ngan RK, Tung SY, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 2015;121:1328–1338.
- Zhang MX, Li J, Shen GP, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51:2587–2595.
- Lewis GD, Holliday EB, Kocak-Uzel E, et al. Intensity-modulated proton therapy for nasopharyngeal carcinoma: decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck. 2016;38(Suppl 1):E1886–E1895.
- Tsang RK, To VS, Ho AC, et al. Early results of robotic assisted nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2015;37:788–793.
- Chan KCA, Woo JKS, King A, et al. Analysis of plasma Epstein–Barr Virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513–522.
- Simo R, Robinson M, Lei M, et al. Nasopharyngeal carcinoma: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S97–S103.
- Gooi Z, Richmon J, Agrawal N, et al. AHNS series – do you know your guidelines? Principles of treatment for nasopharyngeal cancer: a review of the National Comprehensive Cancer Network guidelines. Head Neck. 2017;39:201–205.