Abstract
Background: Over the past 40 years the Danish Breast Cancer Cooperative Group (DBCG) has made significant contributions to improve outcome and to make treatment of patients with early breast cancer more tolerable through nationwide and international trials evaluating loco-regional and systemic treatments. These trials have been instrumental to establish standards for the treatment of early breast cancer.
Methods: The DBCG 82 trials had a global impact by documenting that the significant gain in loco-regional recurrence from postmastectomy radiation added to systemic therapy was associated with a reduction in distant recurrence and mortality in high-risk pre- and postmenopausal patients. The DBCG trials comparing breast conserving surgery and radiotherapy with mastectomy and more recently the trial of internal mammary node irradiation also had a major impact of practice. The trials initiated by the DBCG 40 years ago on tamoxifen and cyclophosphamide based chemotherapy became instrumental for the development of adjuvant systemic therapy not only due to their positive results but by sharing these important data with other members of the Early Breast Cancer Trialist’ Collaborative Group (EBCTCG). Trials from the DBCG have also been important for highlighting the relative importance of anthracyclines and taxanes in the adjuvant setting. Furthermore, DBCG has made a major contribution to the development of aromatase inhibitors and targeted adjuvant treatment for human epidermal growth factor receptor 2 positive breast cancers.
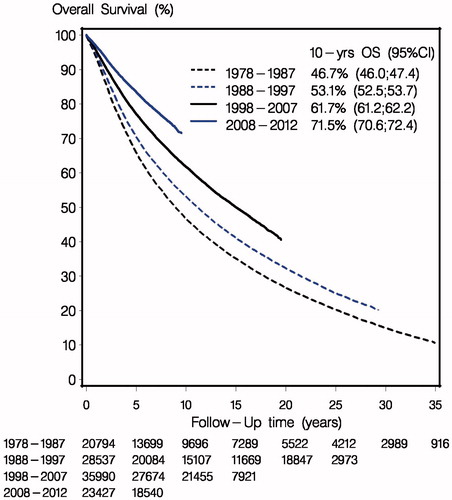
Results: The substantial impact of these treatment improvements is illustrated by a 46.7% 10-year overall survival of early breast cancer patients treated in 1978–1987 compared to 71.5% for patients treated 2008–2012.
Conclusions: The trials conducted and implemented by the DBCG appear to have a major impact on the substantial survival improvements in breast cancer.
Introduction
This paper describes how mortality has improved among Danish breast cancer patients in conjunction with improved quality of loco-regional and systemic therapeutic interventions obtained by the major scientific contributions by Danish Breast Cancer Cooperative Group (DBCG) to clinical research.
Loco-regional treatment studies
Mastectomy and radiotherapy
In the early 1970s the Danish standard treatment of early breast cancer patients was simple mastectomy followed by loco-regional radiotherapy as demonstrated in the Copenhagen Breast Cancer study, where patients were randomly allocated to simple mastectomy followed by radiotherapy or extended radical mastectomy [Citation1–4]. In the DBCG 77 trials the standard treatment was total mastectomy and axillary sampling with the addition of loco-regional radiotherapy in high-risk patients (node-positive and/or invasion to the pectoral fascia and/or T3 or T4 tumors). No systemic treatment was recommended. Orthovoltage was applied ad modum McWhirter, and in 4 out of 5 Danish departments hypofractionation was used due to shortage of radiation capacity [Citation5]. The hypofractionation schedule was a minimum dose of 36.6 Gy in 12 fractions, 2 fractions weekly, or 40.92 Gy/22 fractions, 5 fractions weekly, based on the Ellis NSD formula [Citation6]. A considerable morbidity from hypofractionated orthovoltage radiotherapy was apparent at the time when the early results emerging from adjuvant systemic treatments promulgated the theory proposed by Fisher, e.g., that breast cancer predominantly is systemic disease [Citation7,Citation8]. Thus, there was a shift away from the Halsted theory of breast cancer being a loco-regional disease, and aggressive surgery/radiation as the only road to cure [Citation9]. The DBCG 82B trial included high-risk premenopausal patients assigned to CMF and DBCG 82C included high-risk postmenopausal patients assigned to one year of tamoxifen, and patients in both trials were randomized to loco-regional radiotherapy versus no radiotherapy. An ethical prerequisite was that in the presence of systemic treatment the patients would not benefit from radiotherapy. As part of the DBCG 82 trial a consensus was reached and implemented nationwide by oncologists specialized in radiotherapy regarding target definition and radiation treatment techniques. The target comprised regional lymph nodes, including the internal mammary nodes (IMN), and chestwall. Electrons was applied against the chestwall plus IMN and the energy was selected according to the distance from skin to pleura by ultrasound, thus the resulting radiation doses to the heart were relatively low. The DBCG 82 trials demonstrated a significant reduction in the risk of loco-regional recurrence and mortality () by postmastectomy radiotherapy, irrespective of menopausal status and number of positive lymph nodes [Citation10–13]. These results were confirmed by the EBCTCG meta-analyzes and have widely been implemented in international guidelines [Citation14,Citation15].
Breast conservation
The DBCG 82 TM (tumorectomy versus mastectomy) trial () compared modified mastectomy, or breast conserving surgery (BCS) with residual breast radiotherapy, or BCS with residual breast and regional node radiotherapy [Citation16,Citation17]. No significant difference was seen between the two treatment groups regarding 10-year recurrence-free and 20-year overall survival, p = .94 and p = .24, respectively. The use of BCS has increased to encompass more than 70% of patients with early breast cancer, as a result of the DBCG 82TM, the associated EBCTCG meta-analysis, earlier diagnosis accomplished by mammography screening, neo-adjuvant systemic treatment, and the use of oncoplastic techniques [Citation18,Citation19]. A population-based study performed by DBCG has documented a high risk of reoperation after BCS for non-palpable breast lesions [Citation20]. Radioactive seed has now been introduced as an alternative to the hook wire localization of non-palpable lesions based on results from a large randomized trial [Citation21].
Fractionation of radiotherapy
The poor results obtained by hypofractionation in the DBCG 77 trials and lack of data supporting superiority of hypofractionation compared to normofractionation, caused a reluctance of the DBCG towards moderate hypofraction in the adjuvant breast cancer setting. In 2002 and 2008, large trials from the UK and Canada testing moderate hypofraction versus normofractionation for breast only radiotherapy showed no difference in local control and a trend towards less late radiation morbidity using hypofractionation [Citation22,Citation23]. However, the patients in these trials were not treated with modern chemotherapy nor boost, and in the Canadian trial patients with large breasts had been excluded. The DBCG HYPO trial was therefore initiated in 2009 to clarify the possible implications from large breast size, use of tumor bed boost following adjuvant anthracyclines and taxanes. In March 2014, the DBCG standard was modified to 40 Gy in 15 fractions for breast only radiotherapy following a safety analysis including 1883 patients randomized in the DBCG HYPO trial to 40 Gy in15 fractions compared to 50 Gy in 25 fractions.
In the UK and NL data from the above mentioned trials were extrapolated to patients treated with loco-regional radiotherapy, however, in DBCG this was not accepted. Thus, in 2015 the DBCG Skagen Trial 1 was initiated to introduce moderately hypofractionated loco-regional radiotherapy by randomizing high-risk patients between 40 Gy/15 fractions versus 50 Gy/25 fractions [Citation24,Citation25]. The primary endpoint was arm lymphedema, and secondary endpoints were other morbidities and pattern of recurrence. As of November 2017 around 1100 patients are included, and the trial is expected to accrue around 3000 patients from Europe and Australia.
Partial breast irradiation
In breast recurrences are most often located close to the original tumor bed and the risk of local recurrence has even in high risk patients decreased over decades [Citation26]. Partial breast irradiation (PBI) consequently emerged as an attractive option to lower the radiation burden in selected patients as recently demonstrated in the UK IMPORT LOW trial [Citation27,Citation28]. From 2009 to 2016 the DBCG PBI trial randomized 882 patients to partial versus whole breast radiotherapy and both groups received 40 Gy/15 fractions using the same technique as in the IMPORT Low trial. Early, yet unpublished, results from the DBCG PBI trial regarding morbidity, the primary endpoint, and risk of recurrence are in line with the IMPORT Low trial, and therefore external beam PBI using 40 Gy/15 fractions has since April 2016 been adopted as a standard for selected patients by DBCG.
Treatment of the axilla
During the past 40 years axillary surgery has changed from axillary sampling to axillary lymph node dissection (ALND) and to sentinel node (SN) procedure. Radiotherapy was recommended to lymph node positive patients in the 77 program and this was reinforced by the results obtained in the DBCG 82 postmastectomy trials [Citation10,Citation11]. In a 20-year period ALND partly replaced axillary node level 1 irradiation in node positive patients but the approach of extending radiotherapy to supraclavicular and IMN has been confirmed by others [Citation29,Citation30]. Concern about the possible harmful effect on the heart by IMN radiotherapy and of anthracyclines led in 2003 through 2014 to avoidance of IMN radiotherapy after left-sided breast cancer while IMN radiotherapy was continued following node positive right-sided breast cancer (). With median 8.9 years follow up an overall survival benefit was shown corresponding to the results in two major trials [Citation29–31]. A meta-analysis of these three studies is in process, but international guidelines already have included IMN radiotherapy in their recommendations to high-risk breast cancer patients.
When the SN procedure was introduced just after the turn of the millennium it primarily saved patients with negative nodes from unnecessary morbidity of ALND, but the AMAROS trial suggested that axillary radiotherapy may be substituted for ALND in patients with a positive SN [Citation32–35]. The need of ALND in node positive patients was recently re-challenged and DBCG joined the Swedish led phase 3 SENOMAC trial to evaluate the safety of omitting ALDN in patients with one or two positive SN [Citation36].
Long-term outcome and morbidity after loco-regional therapy
The results obtained by BCS and radiotherapy in the DBCG 82TM trial were confirmed in a successive DBCG 89 cohort with similar recurrence and mortality at 20 years [Citation37]. In a small long-term study with a median of 12 years follow-up 88% of the patients were satisfied with their cosmetic outcome, and poor cosmesis was correlated with use of chemotherapy, large breast size and smoking [Citation38]. Local recurrences continued to occur up to 20 years after diagnosis, and a local recurrence in patients <45 years significantly increased the risk of breast cancer mortality, whilst local recurrence among older patients did not [Citation39]. Patients who following a local recurrence developed distant failure could not be identified by classical histopathological parameters or approximated intrinsic subtypes [Citation40]. Efforts to predicting the risk of late morbidity are being continued as part of the DBCG HYPO and PBI trials.
A seven gene profile predicting gain from radiotherapy has been developed using tumor tissue from patients in the DBCG 82 trials and validated in an independent data set [Citation41,Citation42]. Further studies are in progress with the goal to select high risk patients for omission of radiotherapy. Late morbidity was evaluated in a subgroup of patients treated in the DBCG 82 trials [Citation43]. A highly significant gain from loco-regional radiotherapy was seen in patients with 1–3 and ≥4 metastatic nodes and translated into a survival benefit [Citation11–13]. Further analyzes showed that the gain from radiotherapy was highly heterogeneous depending on immunohistochemical approximated intrinsic subtypes [Citation44].
Very severe late treatment related morbidities are heart disease and second cancer. With 12 years median follow up the DBCG 82 trial did not indicating an increased risk of radiation induced heart disease, but extensive research on heart disease in Danish and Swedish breast cancer patients documented a dose–response relationship between mean heart radiation dose and risk of major coronary event [Citation45–47]. Recently a hypothesis was proposed correlating smoking and anthracyclines with a very high risk of radiation induced heart disease [Citation48,Citation49]. Second cancer has also been intensely investigated in patients treated according to DBCG guidelines, and second lung cancer is by far the largest risk, the magnitude is around 1:200 in every irradiated breast cancer patient [Citation50]. The results showed that >90% of those patients developing second lung cancer were smokers.
Systemic treatment
Adjuvant endocrine treatment
Along with its metabolites, tamoxifen competes with estrogens for binding to the estrogen receptor (ER) and the early results of a CBCT study suggested adjuvant tamoxifen could reduce breast cancer recurrence and prolong survival following surgery of early breast cancer [Citation51]. The benefits from adjuvant tamoxifen observed in postmenopausal high-risk (node positive and/or T3) patients were validated in the DBCG 77C trial (). One year of tamoxifen 30 mg daily reduced the risk of recurrence and mortality in patients with ER positive cancers and no benefit was observed in patients with ER negative and PR positive breast cancer [Citation52,Citation53]. One year of tamoxifen 30 mg daily, the standard in the DBCG 89C trial (), was not inferior to two years of tamoxifen, and a sequence of tamoxifen for 6 months followed by megestrol acetate for six months in postmenopausal high-risk patients [Citation54]. When the Swedish Breast Group trial in 1996 published a beneficial effect from extending tamoxifen from two to five years participants in DBCG 89C still on treatment were offered to extend treatment which may have biased the overall results [Citation55].
Table 1. Loco-regional treatment, 10-year loco-regional recurrence, disease-free and overall survival.
Table 2. Adjuvant endocrine treatment.
The DBCG 82B trial () was in pre- and peri-menopausal high-risk patients unable to demonstrate a reduction in the risk of recurrence or mortality from adding one year of tamoxifen to nine cycles of four-weekly intravenous CMF [Citation56]. Corresponding to our results, the first overview published by the EBCTCG in 1988 was unable to demonstrate a benefit from tamoxifen in patients younger than 50 years [Citation57]. With longer treatment duration and follow-up, the benefit of tamoxifen was however by the EBCTCG shown to be largely independent of age, nodal status, and prior administration of chemotherapy and five years of tamoxifen was introduced as a DBCG standard in 1998 [Citation58,Citation59]. The results from aTTOM and ATLAS demonstrated a further incremental benefit from extending tamoxifen to 10 years [Citation60,Citation61]. While tamoxifen in the eighties was considered less effective in premenopausal patients, chemotherapy was thought to be particular effective and the prevailing view at that time was that this was due to ovarian function suppression (OFS) by chemotherapy [Citation62]. This hypothesis was to some degree confirmed in DBCG 89B that showed a similar DFS and mortality from OFS and CMF [Citation63]. In an exploratory subset analysis the treatment effect largely seemed independent of age, nodal status, tumor size, histological type, malignancy grade, and PR status. However, in the subset with discordant hormone receptor status (either ER or PR negative tumors), CMF resulted in a significant reduction of DFS events and mortality. Several trials support that OFS either alone or in combination with tamoxifen improve outcome similarly to what is achieved with CMF and anthracycline based chemotherapy [Citation64]. Combined the SOFT and TEXT trials demonstrated that OFS with either tamoxifen or an aromatase inhibitor (AI) may lower the risk of recurrence in high-risk premenopausal hormone receptor positive breast cancer patients [Citation65,Citation66]. The cohort study linked to the DBCG 89B trial implied a long-term detrimental effect following OFS corresponding to the data of a long term follow-up of 30,000 participants in the Nurses’ Health Study showing an increased mortality despite a reduced risk of breast- and ovarian cancer from ovariectomy in conjunction with hysterectomy due to benign disease [Citation67,Citation68].
In postmenopausal patients aromatase inhibitors have been evaluated in several large trials, and five years of an aromatase inhibitor further reduces mortality by 15% compared to five years of tamoxifen [Citation69]. The breast international group (BIG) 1–98 study compared five years of tamoxifen with five years of letrozole or the two drugs in sequence (each for two to three years) in postmenopausal patients (). Initial treatment with letrozole reduced the risk of recurrence and mortality [Citation70,Citation71]. The intergroup exemestane study (IES) recruited postmenopausal women who after receiving adequate local and adjuvant systemic therapy remained free of disease after two to three years of tamoxifen (). Switch to exemestane for the remainder of five years endocrine treatment reduced the risk of recurrence and mortality [Citation72,Citation73]. A pronounced benefit of upfront aromatase inhibition has been shown in patients with a lobular histology and in patients at a high risk of relapse [Citation74,Citation75]. A comparable result from letrozole and anastrozole was obtained in the Femara versus Anastrozole Clinical Evaluation (FACE) trial () and from exemestane and anastrozole in NCIC MA.27 [Citation76,Citation77]. Ongoing clinical trials are evaluating the role of extending endocrine treatment after five years of an aromatase inhibitor and in the meanwhile the study of letrozole extension (SOLE) has shown () that continuous extended treatment may be substituted by intermittent aromatase inhibition [Citation78]. Only a small group of postmenopausal patients, i.e., node negative patients older than 60 with grade 1 tumors ≤10 mm, will without systemic treatment achieve an age-appropriate survival [Citation79].
Adjuvant HER2 targeted treatment
The addition of a one-year course of trastuzumab to chemotherapy in human epidermal growth factor 2 (HER2) positive disease significantly reduced disease recurrences and mortality in several trials including HERceptin Adjuvant (HERA) trial [Citation80–82]. HERA enrolled 5081 patients; hereof 133 by DBCG, from 2001 to 2005 were adjuvant trastuzumab was introduced as a standard (). DBCG contributed to international but unfruitful efforts to further improve outcome by extending duration of trastuzumab to two years in HERA and concurrent or sequential addition of lapatinib in the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation (ALTTO) trial [Citation83,Citation84]. DBCG investigators enrolled 87 patients in the Adjuvant Pertuzumab and Herceptin in Initial Therapy (APHINITY) trial, which showed a statistically significant reduction in DFS events from the addition of pertuzumab to trastuzumab-based adjuvant therapy [Citation85] but the clinical benefit has yet to be established. Finally, the EXTEnded NEratinib Trial (ExteNET) had participation of 112 patients though DBCG and showed that one year of neratinib, an irreversible tyrosine kinase inhibitor of HER1, HER2 and HER4, compared to placebo further significantly improved DFS when given after one year of trastuzumab-based (neo)adjuvant therapy in HER2-positive breast cancer [Citation86]. One year of trastuzumab added to adjuvant chemotherapy remains standard of care in patients with early-stage HER2-positive breast cancer but neratinib may become an option to some patients [Citation87].
Table 3. Adjuvant HER2 targeted treatment.
Adjuvant chemotherapy
The achievements of the DBCG with adjuvant chemotherapy has recently been reviewed [Citation88]. In brief, the DBCG 77B trial () showed significant and clinical important reduction in the risk of recurrence and mortality from single agent oral cyclophosphamide and from CMF in premenopausal patients with high-risk (node-positive or T3) breast cancer [Citation89]. Adjuvant CMF was in 1982 selected as a standard in high-risk premenopausal breast cancer by DBCG and subsequently in 1985 by the first NIH Consensus development Conference [Citation90]. In a retrospective analysis patients with core-basal and luminal B breast cancers appeared to derive the largest benefit from cyclophosphamide-based chemotherapy [Citation91]. No apparent benefit was observed from the addition of CMF to tamoxifen among postmenopausal high-risk breast cancer patients in an early analysis of DBCG trial 82C [Citation92] and no clear benefit was shown from adjuvant chemotherapy in the first Oxford overview published in 1988 [Citation57]. A differential benefit however was shown in patients with ER negative breast cancer and adjuvant chemotherapy was in 1989 extended to high-risk postmenopausal patients younger than 70 with ER negative breast cancer. Furthermore, invasive ductal carcinomas with malignancy grade 2 and 3 were irrespective of tumor size and lymph node status included in the premenopausal high-risk group by 1989.
Table 4. Adjuvant chemotherapy.
With longer follow-up CMF was associated with a significant improvement in DFS [Citation92,Citation93]. Apart from menopausal status, the DBCG 77B and 82C trials had identical selection criteria, but while 77B trial used classic CMF with oral cyclophosphamide a four-weekly intravenous CMF regimen was used in DBCG 82B and C trials, and a three-weekly CMF regimen was used in the succeeding DBCG 89B and D trials. A population-based DBCG study demonstrated that shifting from classical CMF in DBCG 77B to four-weekly or three-weekly i.v. CMF was associated with a 30% increased risk of a DFS event [Citation94]. Furthermore, the four-weekly regimen as used in DBCG 82B was associated with a 40% increase in mortality.
Danish breast cancer cooperative group 89D showed an incremental reduction in recurrence and mortality from substituting methotrexate with epirubicin [Citation95]. The advantage of anthracycline-containing three-drug combinations over CMF was confirmed by others and by meta-analysis conducted by EBCTCG, while standard AC (doxorubicin and cyclophophamide) or EC (epirubicin and cyclophosphamide) for four cycles was not superior to classic CMF [Citation96]. HER2 and TOP2A were assessed retrospectively in 767 of the 980 Danish patients included in DBCG 89D. Topoisomerase IIα, the enzyme encoded by TOP2A, is a direct target for anthracyclines and essential for resolving topological DNA constraints [Citation97,Citation98]. Alteration of TOP2A copy number was in 89D associated with an incremental benefit from epirubicin [Citation99]. This was confirmed in a prospectively planned joint analysis of 89D and four other phase three trials [Citation100] but only a trend toward greater benefit was show for patients with HER2-amplified tumors. The DBCG 07-READ compared six cycles of docetaxel and cyclophosphamide with three cycles of epirubicin and cyclophosphamide followed by three cycles of docetaxel and confirmed no overall benefit from adjuvant epirubicin in patients with early and TOP2A-normal breast cancer [Citation101]. Other mechanisms of action have been proposed including protection from apoptosis by tissue inhibitor of matrix metalloproreinases-1 (TIMP-1). A highly significant interaction was shown in 89D between epirubicin and a classifier constructed by combining lack of TIMP-1 expression and/or TOP2A alteration [Citation102].
In BIG 2-98 () a further reduction in the risk of recurrence was obtained from adding a taxane to sequential anthracycline and CMF but not from substitution of cycles of cyclophosphamide and doxorubicin with docetaxel and doxorubicin [Citation103]. Similarly a further reduction in breast cancer mortality appeared in the EBCTCG meta-analysis from the addition of a taxane to a standard AC, while the substitution of cycles or drugs with a taxane was not associated with a reduction in mortality [Citation96]. ECOG 1199 in a factorial 2 by two design showed a superior benefit from weekly paclitaxel compared to three-weekly and from three-weekly docetaxel compared to weekly, and overall no difference between docetaxel and paclitaxel [Citation104].
Results
Overall survival is in presented according to period of diagnosis for all patients with operable invasive breast cancer, irrespective of the received loco-regional and systemic treatment. A major and clinical important improvement in prognosis according to period of diagnosis is apparent with a decrease in 10 year mortality from 53.3% in 1978–1987 to 28.5% for those diagnosed in 2007–2015 ().
Figure 1. Overall survival (OS) according to period of diagnosis. All patients, irrespective of adjuvant treatment.
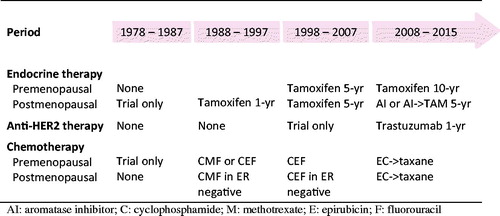
A temporary setback in the otherwise continuous improvement in prognosis occurred in the second half of the 1978–1987 period as a consequence of a less effective adjuvant CMF regimen and omission of postmastectomy radiotherapy in patients randomized to the control group in the DBCG 82B&C trials. The improvements obtained in the succeeding periods can hardly be attributed to a single treatment or trial (). The proportion of patients receiving adjuvant treatment increased from none to more than 80%, the proportion of patients who received chemotherapy in combination with endocrine therapy and/or trastuzumab increased and the distinct treatment regimens evolved as described in and the preceding sections.
Discussion
In this nationwide and population-based study we have demonstrated a significant improvement of the prognosis following early breast cancer in four successive decades. Furthermore, the present study indicates that the substantial reduction in mortality from to 53.3% to 28.5% in first 10 years after breast cancer is closely connected to results obtained in clinical trials and in particular to those obtained by the Danish Breast Cancer Cooperative Group nationwide or in internal collaboration. In a study accompanying our study in this issue a quite fast implementation of guideline modification is shown over the last decade and equivalent results have previously been shown for the preceding three decades [Citation105,Citation106].
Several issues should be considered when interpreting this study. First, the population-based design of our clinical database and the prospective and comprehensive registration of patients and treatments minimized the risk of bias. Second, patients above 70 at diagnosis of breast cancer and patients with multimorbidity were not included in phase 3 trials and results from clinical trials may not have been implemented fully in non-eligible patients. Third, life-expectancy has gradually been increasing during the last four decades and may in part explain our results. Finally, earlier diagnosis and alterations in the biology of the disease may have contributed to reductions in mortality.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kaae S, Johansen H. Does simple mastectomy followed by irradiation offer survival comparable to radical procedures? Int J Radiat Oncol Biol Phys. 1977;2:1163–1166.
- McWhirter R. Simple mastectomy and radiotherapy in the treatment of breast cancer. Br J Radiol. 1955;28:128–139.
- Dahl-Iversen E, Toiassen T. Radical mastectomy with parasternal and supraclavicular dissection for mammary carcinoma. Ann Surg. 1963;157:170–173.
- Johansen H, Kaae S, Johansen H, et al. Extended radical mastectomy versus simple mastectomy followed by radiotherapy in primary breast cancer. A fifty-year follow-up to the Copenhagen breast cancer randomised study. Acta Oncol. 2008;47:633–638.
- McWhirter R. The value of simple mastectomy and radiotherapy in the treatment of cancer of the breast. BJR. 1948;21:99–610.
- Ellis F. Dose, time and fractionation: A clinical hypothesis. Clin Radiol. 1969;20:1–7.
- Bentzen S, Overgaard M. Early and late normal tissue injury after postmastectomy radiotherapy. Recent Results Cancer Res. 1993;130:59–78.
- Fisher B. Laboratory and clinical research in breast cancer – a personal adventure: the David A. Karnofsky memorial lecture. Cancer Res. 1980;40:3863–3874.
- Halsted WS. I. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;46:1.
- Overgaard M, Hansen PS, Overgaard J, Danish Breast Cancer Cooperative Group 82b Trial, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949–955.
- Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish breast cancer cooperative group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648.
- Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82:247–253.
- Nielsen HM, Overgaard M, Grau C, et al. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish breast cancer cooperative group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24:2268–2275.
- McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135.
- Curigliano G, Burstein HJ, P Winer E, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28:1700–1712.
- Blichert-Toft M, Brincker H, Andersen JA, et al. A Danish randomized trial comparing breast-preserving therapy with mastectomy in mammary carcinoma. Preliminary results. Acta Oncol. 1988;27:671–677.
- Blichert-Toft M, Nielsen M, Düring M, et al. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008;47:672–681.
- Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106.
- Christiansen PM, Carstensen SL, Ejlertsen B, et al. Breast conserving surgery versus mastectomy: overall and relative survival – a population based study by the Danish breast cancer cooperative group. Acta Oncol. 2017 [Nov 23]; [7 p.]. DOI:10.1080/0284186X.2017.1403042
- Langhans L, Jensen MB, Talman MM, et al. Reoperation rates in ductal carcinoma in situ vs invasive breast cancer after wire-guided breast-conserving surgery. JAMA Surg. 2017;152:378–384.
- Langhans L, Tvedskov TF, Klausen TL, et al. Radioactive seed localization or wire-guided localization of nonpalpable invasive and in situ breast cancer: a randomized, multicenter, open-label trial. Ann Surg. 2017;266:29–35.
- Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143–1150.
- Bentzen SM, Agrawal RK, Aird EG, START Trialists’ Group, et al. The UK standardisation of breast radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098–1107.
- Nielsen HM, Friis RB, Linnet S, et al. Loco-regional morbidity after breast conservation and axillary lymph node dissection for early breast cancer with or without regional nodes radiotherapy, perspectives in modern breast cancer treatment: the Skagen Trial 1 is active. Acta Oncol. 2017;56:713–718.
- Francolini G, Thomsen MS, Yates ES, et al. Quality assessment of delineation and dose planning of early breast cancer patients included in the randomized Skagen Trial 1. Radiother Oncol. 2017;123:282–287.
- Poortmans PM, Arenas M, Livi L. Over-irradiation. Breast. 2017;31:295–302.
- Offersen BV, Overgaard M, Kroman N, et al. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol. 2009;90:1–13.
- Coles CE, Griffin CL, Kirby AM, IMPORT Trialists, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UKIMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390:1048–1060.
- Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–327.
- Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:1878–1879.
- Thorsen LB, Offersen BV, Danø H, et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. 2016;34:314–320.
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310.
- Friis E, Galatius H, Garne JP. Organized nation-wide implementation of sentinel lymph node biopsy in Denmark. Acta Oncol. 2008;47:556–560.
- Christiansen P, Friis E, Balslev E, et al. Sentinel node biopsy in breast cancer: five years experience from Denmark. Acta Oncol. 2008;47:561–568.
- Husted Madsen A, Haugaard K, Soerensen J, et al. Arm morbidity following sentinel lymph node biopsy or axillary lymph node dissection: a study from the Danish breast cancer cooperative group. Breast. 2008;17:138–147.
- de Boniface J, Frisell J, Andersson Y, et al. Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer. 2017;17:379.
- Lyngholm CD, Laurberg T, Alsner J, et al. Failure pattern and survival after breast conserving therapy. Long-term results of the Danish Breast Cancer Group (DBCG) 89 TM cohort. Acta Oncol. 2016;55:983–992.
- Lyngholm CD, Christiansen PM, Damsgaard TE, et al. Long-term follow-up of late morbidity, cosmetic outcome and body image after breast conserving therapy. A study from the Danish Breast Cancer Cooperative Group (DBCG). Acta Oncol. 2013;52:259–269.
- Laurberg T, Lyngholm CD, Christiansen PM, et al. Long-term age-dependent failure pattern after breast-conserving therapy or mastectomy among Danish lymph-node-negative breast cancer patients. Radiother Oncol. 2016;120:98–106.
- Laurberg T, Alsner J, Tramm T, et al. Impact of age, intrinsic subtype and local treatment on long-term locol-regional recurrence and breast cancer mortality among low-risk breast cancer patients. Acta Oncol. 2017;56:59–67.
- Tramm T, Kyndi M, Myhre S, et al. Relationship between the prognostic and predictive value of the intrinsic subtypes and a validated gene profile predictive of loco-regional control and benefit from post-mastectomy radiotherapy in patients with high-risk breast cancer. Acta Oncol. 2014;53:1337–1346.
- Tramm T, Mohammed H, Myhre S, et al. Development and validation of a gene profile predicting benefit from postmastectomy radiotherapy in patients with high-risk breast cancer: a study of gene expression in the DBCG 82 b&c cohort. Clin Can Res. 2014;20:5272–5280.
- Højris I, Andersen J, Overgaard M, et al. Late treatment-related morbidity in breast cancer patients randomized to postmastectomy radiotherapy and systemic therapy versus systemic therapy alone. Acta Oncol. 2000;39:355–372.
- Kyndi M, Overgaard M, Nielsen HM, et al. High local recurrence risk is not associated with large survival reduction after postmastectomy radiotherapy in high-risk breast cancer: a subgroup analysis of DBCG 82 b&c. Radiother Oncol. 2009;90:74–79.
- Højris I, Overgaard M, Christensen JJ, et al. Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: analysis of DBCG 82b and 82c randomized trials. Lancet. 1999;354:1425–1430.
- McGale P, Darby S, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–175.
- Darby S, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998.
- Taylor C, Correa C, Duane F, et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. JCO. 2017;35:1641–1649.
- Rehammar JC, Jensen MB, McGale P, et al. Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977–2005. Radiother Oncol. 2017;123:299–305.
- Grantzau T, Thomsen MS, Vaeth M, et al. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother Oncol. 2014;111:366–373.
- Jensen MB, Krarup JF, Palshof T, et al. Two years of tamoxifen or no adjuvant systemic therapy for patients with high-risk breast cancer: long-term follow-up of the Copenhagen breast cancer trial. Acta Oncol. 2017 [Nov 22]; [5 p.]. DOI:10.1080/0284186X.2017.1400179
- Knoop AS, Bentzen SM, Nielsen MM, et al. Value of epidermal growth factor receptor, HER2, p53, and steroid receptors in predicting the efficacy of tamoxifen in high-risk postmenopausal breast cancer patients. JCO. 2001;19:3376–3384.
- Knoop AS, Laenkholm AV, Jensen MB, Danish Breast Cancer Cooperative Group, et al. Estrogen receptor, Progesterone receptor, HER2 status and Ki67 index and responsiveness to adjuvant tamoxifen in postmenopausal high-risk breast cancer patients enrolled in the DBCG 77C trial. Eur J Cancer. 2014;50:1412–1421.
- Andersen J, Kamby C, Ejlertsen B, et al. Tamoxifen for one year versus two years versus 6 months of Tamoxifen and 6 months of megestrol acetate: a randomized comparison in postmenopausal patients with high-risk breast cancer (DBCG 89C). Acta Oncol. 2008;47:718–724.
- Swedish Breast Cancer Cooperative Group. Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst. 1996;88:1543–1549.
- Andersson M, Kamby C, Jensen MB, et al. Tamoxifen in high-risk premenopausal women with primary breast cancer receiving adjuvant chemotherapy. Report from the Danish breast cancer co-operative group DBCG 82B trial. Eur J Cancer. 1999;35:1659–1666.
- Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681–1692.
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–1467.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784.
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816.
- Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. 2013;31(15_Suppl):5. DOI:10.1200/jco.2013.31.15_suppl.5
- Brincker H, Rose C, Rank F, et al. Evidence of a castration-mediated effect of adjuvant cytotoxic chemotherapy in premenopausal breast cancer. JCO. 1987;5:1771–1778.
- Ejlertsen B, Mouridsen HT, Jensen MB, et al. Similar efficacy for ovarian ablation compared with cyclophosphamide, methotrexate, and fluorouracil: from a randomized comparison of premenopausal patients with node-positive, hormone receptor-positive breast cancer. JCO. 2006;24:4956–4962.
- Cuzick J, Ambroisine L, Davidson N, et al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007;369:1711–1723.
- Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118.
- Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446.
- Ejlertsen B, Jensen MB, Mouridsen HT, et al. DBCG trial 89B comparing adjuvant CMF and ovarian ablation: similar outcome for eligible but non-enrolled and randomized breast cancer patients. Acta Oncol. 2008;47:709–717.
- Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709–716.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352.
- Mouridsen H, Giobbie-Hurder A, Goldhirsch A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:766–776.
- Regan MM, Neven P, Giobbie-Hurder A, BIG 1-98 Collaborative Group; International Breast Cancer Study Group (IBCSG), et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol. 2011;12:1101–1108.
- Coombes RC, Hall E, Gibson LJ, Intergroup Exemestane Study, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092.
- Bliss JM, Kilburn LS, Coleman RE, et al. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol. 2012;30:709–717.
- Metzger Filho O, Giobbie-Hurder A, Mallon E, et al. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the big 1-98 trial. JCO. 2015;33:2772–2779.
- Rasmussen BB, Regan MM, Lykkesfeldt AE, et al. Adjuvant letrozole versus tamoxifen according to centrally-assessed ERBB2 status for postmenopausal women with endocrine-responsive early breast cancer: supplementary results from the BIG 1-98 randomised trial. Lancet Oncol. 2008;9:23–28.
- Smith I, Yardley D, Burris H, et al. Comparative efficacy and safety of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor-positive, node-positive early breast cancer: final results of the randomized phase III femara versus anastrozole clinical evaluation (FACE) trial. JCO. 2017;35:1041–1048.
- Goss PE, Ingle JN, Pritchard KI, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27–a randomized controlled phase III trial. JCO. 2013;31:1398–1404.
- Colleoni M, Luo W, Karlsson P, et al. SOLE (Study of Letrozole Extension): a phase III randomized clinical trial of continuous vs intermittent letrozole in postmenopausal women who have received 4–6 years of adjuvant endocrine therapy for lymph node-positive, early breast cancer (BC). J Clin Oncol. 2017;35(15_Suppl):503. DOI:10.1200/JCO.2017.35.15_suppl.503
- Christiansen P, Bjerre K, Ejlertsen B, et al. Mortality rates among early-stage hormone receptor-positive breast cancer patients: a population-based cohort study in Denmark. J Natl Cancer Inst. 2011;103:1363–1372.
- Gianni L, Dafni U, Gelber RD, Herceptin Adjuvant (HERA) Trial Study Team, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244.
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. JCO. 2014;32:3744–3752.
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283.
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205.
- Piccart-Gebhart M, Holmes E, Baselga J, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol. 2016;34:1034–1042.
- von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131.
- Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:367–377.
- Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2017 [Nov 13]. DOI:10.1016/S1470-2045(17)30717-9
- Ejlertsen B. Adjuvant chemotherapy in early breast cancer. Dan Med J. 2016;63:pii:B5222.
- Ejlertsen B, Mouridsen HT, Jensen MB, et al. Cyclophosphamide, methotrexate, and fluorouracil; oral cyclophosphamide; levamisole; or no adjuvant therapy for patients with high-risk, premenopausal breast cancer. Cancer. 2010;116:2081–2089.
- National Institutes of Health Consensus Development Conference Statement: Adjuvant Chemotherapy for Breast Cancer. September 9–11, 1985. CA Cancer J Clin. 1986;36:42–47.
- Nielsen TO, Jensen MB, Burugu S, et al. High-risk, premenopausal luminal A breast cancer patients derive no benefit from adjuvant cyclophosphamide-based chemotherapy: Results from the DBCG77B clinical trial. Clin Cancer Res. 2017;23:946–953.
- Mouridsen HT, Rose C, Overgaard M, et al. Adjuvant treatment of postmenopausal patients with high risk primary breast cancer. Results from the Danish adjuvant trials DBCG 77 C and DBCG 82 C. Acta Oncol. 1988;27:699–705.
- Ejlertsen B, Jensen MB, Elversang J, et al. One year of adjuvant tamoxifen compared with chemotherapy and tamoxifen in postmenopausal patients with stage II breast cancer. Eur J Cancer. 2013;49:2986–2994.
- Ejlertsen B, Mouridsen HT, Jensen MB. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in premonopausal patients with node-positive breast cancer: indirect comparison of dose and schedule in DBCG trials 77, 82, and 89. Acta Oncol. 2008;47:662–671.
- Ejlertsen B, Mouridsen HT, Jensen MB, et al. Improved outcome from substituting methotrexate with epirubicin: results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer. 2007;43:877–884.
- Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444.
- Baxter J, Sen N, Martínez VL, et al. Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science. 2011;331:1328–1332.
- Kellner U, Sehested M, Jensen PB, et al. Culprit and victim – DNA topoisomerase II. Lancet Oncol. 2002;3:235–243.
- Knoop AS, Knudsen H, Balslev E, et al. Retrospective analysis of topoisomerase IIa amplifications and deletions as predictive markers in primary breast cancer patients randomly assigned to cyclophosphamide, methotrexate, and fluorouracil or cyclophosphamide, epirubicin, and fluorouracil: Danish Breast Cancer Cooperative Group. J Clin Oncol. 2005;23:7483–7490.
- Di Leo A, Desmedt C, Bartlett JM, et al. HER2 and TOP2A as predictive markers for anthracycline-containing chemotherapy regimens as adjuvant treatment of breast cancer: a meta-analysis of individual patient data. Lancet Oncol. 2011;12:1134–1142.
- Ejlertsen B, Tuxen MK, Jakobsen EH, et al. Adjuvant cyclophosphamide and docetaxel with or without epirubicin for early TOP2A-normal breast cancer: DBCG 07-READ, an open-label, phase III, randomized trial. JCO. 2017;35:2639–2646.
- Ejlertsen B, Jensen MB, Nielsen KV, et al. HER2, TOP2A, and TIMP-1 and responsiveness to adjuvant anthracycline-containing chemotherapy in high-risk breast cancer patients. JCO. 2010;28:984–990.
- Oakman C, Francis PA, Crown J, et al. Overall survival benefit for sequential doxorubicin-docetaxel compared with concurrent doxorubicin and docetaxel in node-positive breast cancer–8-year results of the breast international group 02-98 phase III trial. Ann Oncol. 2013;24:1203–1211.
- Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671.
- Jensen MB, Ejlertsen B, Mouridsen HT, et al. Improvements in breast cancer survival between 1995 and 2012 in Denmark: the importance of earlier diagnosis and adjuvant treatment. Acta Oncol. 2016;55:24–35.
- Møller S, Jensen MB, Ejlertsen B, et al. The clinical database and the treatment guidelines of the Danish breast cancer cooperative group (DBCG); its 30-years experience and future promise. Acta Oncol. 2008;47:506–524.