Introduction
With the advent of the anti-programmed cell death-1 (anti-PD-1) and anti-programmed cell death ligand-1 (anti-PD-L1) therapies, the treatment landscape for non-small cell lung cancer (NSCLC) has seen a paradigm shift, resulting in improved response rates and durability in survival [Citation1]. By targeting the PD-1/PD-L1 pathway, these antibodies revert the immune tolerance of the tumor, making it vulnerable to an attack by the host immune system [Citation2,Citation3]. Despite the advances in disease control witnessed in the era of immunotherapy, the response to ICB continues to be inconsistent. Moreover, due to the dynamic nature of the immune response in vivo, the discovery of biomarkers predicting outcomes to ICB has been challenging. Currently, a plethora of putative markers are being investigated to understand their role in determining tumor immunogenicity and predicting the outcome to ICB [Citation4]. Among these, the PD-L1 expression on tumors is considered to be one of the leading determinants of the response to ICB [Citation5]. However, as we know, its utility has been plagued by controversy regarding the lack of standardized testing methods, and definition of positivity [Citation6,Citation7]. Hence, the research efforts directed at identifying reliable biomarkers to facilitate recognition of patients most amenable to benefit from ICB are gaining importance.
By fostering a microenvironment that is favorable for tumor growth and propagation, chronic inflammation constitutes an essential facet of the neoplastic process [Citation8,Citation9]. Historically, certain peripheral blood-based biomarkers that serve as a surrogate measure for the degree of systemic inflammation have been used to both prognosticate patients as well as predict outcomes to treatments. One such marker, the modified Glasgow prognostic score (mGPS) which is a composite of biomarkers representative of inflammation, i.e., C-reactive protein (CRP) and serum albumin, has shown prognostic value in patients with a multitude of malignancies [Citation10,Citation11]. While there is also data suggesting the predictive value of mGPS in stage IIIB/IV NSCLC for platinum-based chemotherapy [Citation12], the role of using mGPS in ICB is unclear. Other candidate markers include the neutrophil to lymphocyte ratio (NLR) and prognostic nutrition index (PNI). Similar to mGPS, NLR and PNI are considered to be readily available parameters crudely corresponding to the degree of systemic inflammation. Both these markers have shown merit as indicators of prognosis in a spectrum of cancers [Citation13,Citation14]. However, like the mGPS, their value as biomarkers in NSCLC patients undergoing ICB is not well studied.
To understand the inter-relationship between inflammation and outcomes in anti-PD-1-treated NSCLC, we initiated a clinical study, the predictive value of the modified Glasgow prognostic score among patients undergoing immunotherapy with PD-1-targeted agents (P-MAIT). Herein, we report the exploratory analysis of P-MAIT examining the co-relation of not only the mGPS but also evaluating the association of readily available candidate inflammatory biomarkers (CRP, NLR, PNI) with survival.
Material and methods
Study design and patient selection
We chose an ambidirectional design for our study. Retrospectively, we identified 87 NSCLC patients treated with anti-PD-1 therapy, i.e., nivolumab (Opdivo, Bristol-Myers Squibb's®) at East Carolina University/Vidant Medical Center from April 2015 to March 2017. We followed this retrospectively identified NSCLC cohort until 31 May 2017, to measure survival outcomes. All these patients had received at least one cycle of nivolumab after progression/relapse on a first-line platinum-based chemotherapy. These patients were part of an ongoing prospectively designed institutional review board approved the biomarker-based study, i.e., P-MAIT. As part of this study, all enrolled patients had baseline inflammatory biomarkers measured on day 0, i.e., the day of immunotherapy initiation and subsequently with each treatment.
Data collection
Clinical information
We gathered retrospective data on patient-specific demographics and clinicopathological characteristics from the electronic medical records. We categorized self-reported smoking history as never (<100 cigarettes), former (>5 pk-yrs, quit >1 year prior) or current smokers. We also collected data with regards to incidence, severity and management of nivolumab induced immune-related adverse events (irAEs). We used common terminology criteria for adverse events (CTCAE; version 4.0) for grading the irAEs.
Peripheral blood-based inflammatory biomarkers
We collected baseline data on serological markers of inflammation, i.e., CRP, albumin, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), NLR, PNI on day 0, i.e., the day of the first dose of anti-PD-1 therapy. The mGPS score was calculated as a composite of serum albumin and CRP protein values: 0 for CRP <10 mg/l, 1 for CRP >10 mg/l and albumin >3.5 g/dL and 2 for CRP >10 mg/l and albumin <3.5 g/dl. NLR was calculated based on the ratio of neutrophil to the lymphocyte count. The PNI was calculated using the following formula: 10 × serum albumin value (g/dl) + 0.005 × peripheral lymphocyte count/mm3.
Objectives
The primary objective of our study was to determine the association of inflammation-based biomarkers, i.e., CRP, NLR, mGPS and PNI with overall survival after immunotherapy (OSI). We defined OSI as the time from anti-PD-1 initiation to death or termination of follow-up. We set the cutoff for OSI estimation as 31 May 2017. As part of the exploratory analysis, we also measured baseline differences in the three biomarkers at anti-PD-1 initiation in relation to clinically meaningful subgroups (SupplementaryTable1).
Statistical analysis
Continuous variables such as CRP, albumin, NLR and PNI, were converted to dichotomous variables using the web-based program by Budczies et al. [Citation15] to enable optimal stratification. Using this online tool, the optimal CRP cutoff was 49.1 mg/l (rounded to 50 mg/l), NLR was 6.5 and PNI was 31.5. This enabled binary partitioning of groups, i.e., CRP ≤50 and >50; NLR ≤6.5 and >6.5; PNI ≤31.5 and >31.5. The cutoff for albumin was set at 3.5 g/dl crudely corresponding with hypoalbuminemia as used in previous studies [Citation16].
Chi-square/Fisher's exact test (as appropriate) was used to determine associations between categorical covariates of interest. Normally distributed continuous variables for a grouping variable were compared using an independent t-test. Paired t-test was used to assess pre- and post-treatment differences. We used the univariate Cox proportional method to determine the association between OSI and covariates that were clinically relevant. From the univariable-level analyses, we identified potential covariates associated with OSI (p<.1). These were then fit into the multivariable Cox proportional hazards model using backward selection to recognize factors that were independently associated with survival [Citation17]. Hazard ratios (HR) with 95% confidence interval (CI) were also calculated using univariate and multivariate analyses. Unadjusted Kaplan–Meier survival curves with log-rank testing were generated to compare the survival. The analysis was conducted using IBM SPSS (version 22.0, SPSS, Chicago, IL) and Graph Pad Prism (version 5.0, Graph Pad Software, La Jolla, CA). All statistical assessments were two-sided. p ≤ .05 was considered as significant.
Results
Patient characteristics
The median age was 64 (range 35–85) with male predominance (64.4%). Ethnicity was Caucasian in a majority of our cohort (63.2%). The predominant histology was adenocarcinoma in 49.4% patients. Stage IV disease was present in 70.1% patients, with the skeletal involvement (54.1%) and liver (27.9%) being the most common metastatic sites. The median albumin level was 3.6 g/dl. The median for CRP, neutrophil count and the lymphocyte count was 22.1 mg/l, 6100/mm3 and 900/mm3, respectively. The median PNI was 36.0 and NLR was 6.0. Other clinical characteristics including differences in the medians of the baseline inflammatory biomarkers in relation to the patient-related clinical/biochemical characteristics are displayed (SupplementaryTable1).
Outcomes
Overall survival after immunotherapy
The median OSI was 8.7 months for the entire cohort. Factors found to be associated with higher mortality in the univariate model with p<.1 were: non-squamous histology, Caucasian ethnicity, CRP >50 mg/L, NLR >6.5, PNI ≤31.5. After including these in the multivariate Cox proportional hazard model using backward elimination, only CRP >50, NLR >6.5 and PNI ≤31.5 were found to be significantly associated with worse OSI ().
Table 1. Factors associated with OSI.
(1) C-reactive protein and OSI
Seventy percent patients had CRP ≤50, while 28.7% had CRP >50 (SupplementaryTable1). At the completion of the entire follow-up period, a significantly higher proportion of patients from the CRP ≤50 group were alive compared to the CRP >50 (49 vs. 16%). CRP >50 had inferior median OSI by the log-rank method in advanced stage NSCLC (2.7 vs. 11 months, p<.01; ).
Figure 1. (a) C-reactive protein (CRP) affecting OSI. (b) Neutrophil to lymphocyte ratio (NLR) affecting OSI. (c) Prognostic nutritional index (PNI) affecting OSI. (d) Modified Glasgow prognostic score (mGPS) affecting OSI. (e) Mean CRP with 95% CI for N = 34: (a) baseline CRP at immunotherapy initiation and (b) CRP at grade III–IV irAEs. Paired t-test for a vs. b is displayed as p < .001.
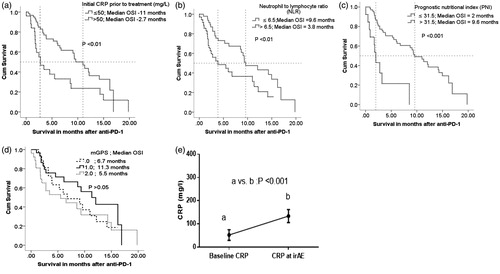
(2) Neutrophil to lymphocyte ratio and OSI
Approximately, 55% patients had NLR ≤6.5 (SupplementaryTable1). Using the log-rank method stratification based on NLR of ≤6.5 vs. >6.5 revealed a significant difference in median OSI (9.6 vs. 3.8 months; p < .01; ).
(3) Prognostic nutrition index and OSI
The optimal cutoff for PNI was designated to be 31.5 based on the method described previously. The majority (78.1%) had PNI >31.5. NSCLC patients with PNI >31.5 were found to have significantly higher median OSI compared to patients with PNI ≤31.5 (; 2 vs. 9.6 months; p < .001).
(4) Modified Glasgow prognostic score and OSI
Based on the cut offs in the mGPS model described above, patients were grouped into three categories, i.e., 0, 1 and 2. Our study population had a roughly similar distribution in the number of patients across all three categories (SupplementaryTable1). Median OSI was highest for mGPS of 1 (11.3 months) followed by 6.7 months for mGPS of 0 and 5.5 months for mGPS of 1 (). No statistical differences were noted between the groups.
Immune-related adverse events and inflammatory biomarkers
In our NSCLC study population, 37% experienced an index irAE ranging from grade II to IV (SupplementaryTable1). We noted a significant increase in CRP at the time of grade II–IV irAEs compared to baseline CRP (; a vs. b). However, there was no statistically significant difference between baseline, i.e., pre-ICB inflammatory biomarkers (CRP, NLR, PNI, mGPS) in patients that had irAEs vs. those that did not (SupplementaryTable1).
Discussion
Markers of systemic inflammation like, CRP, mGPS, NLR and PNI have been shown to have a prognostic relevance in a multitude of malignancies that is independent of clinicopathological characteristics [Citation10,Citation13,Citation14,Citation18]. Furthermore, emerging data from recent studies have tried to validate the predictive utility of these readily available inflammatory biomarkers in NSCLC patients treated with ICB [Citation19–25]. To the best of our knowledge, our data comprising of 87 patients with NSCLC initiated on ICB, is one of the most extensive North American experiences aimed at discerning the relationship between OSI and readily available peripheral blood-based candidate inflammatory biomarkers. Similar to some prior observations in non-immunotherapy settings, we have attempted to demonstrate an interdependence between baseline systemic inflammation and survival with anti-PD-1 treatment. All patients in our study had progressive or relapsed disease following platinum-based chemotherapy.
As a primary outcome, our study was able to demonstrate a significant association of baseline CRP, NLR and PNI with OSI for NSCLC patients treated with anti-PD-1 therapy. These markers (CRP, NLR and PNI) indirectly represent essential cellular components of the host immunome that are pivotal to mechanisms regulating the degree of the effectiveness of an immune-mediated anti-tumor response. For example, at the molecular level, an elevated CRP has been found to inversely correlate with the presence of CD-4 infiltrating lymphocytes in the tumor milieu, which in-turn represents a poor prognostic factor [Citation26]. In the clinical context, the role of CRP as a potential biomarker and its relationship with cancer outcomes has been elucidated previously [Citation18], with recent corroboration in melanoma treated with ICB [Citation27]. Similar to CRP, pretreatment NLR and PNI are considered to be readily available parameters corresponding crudely to the degree of host immune inflammation and have been shown to stratify outcomes in a spectrum of cancers [Citation13,Citation14]. Pre-clinical studies provide a bulk of evidence to support the pro-metastatic role of neutrophils [Citation28]. Neutrophils can induce immunosuppression via an array of mechanisms including inhibition of T-cell mediated antitumor response by secreting factors such as arginase, reactive oxygen species and nitric oxide [Citation29]. Moreover, albumin and lymphocytes that constitute the PNI, as components of the host defense are known to modulate the immune system to generate an anti-cancer response [Citation9,Citation16].
More recently, emerging data looking into the usefulness of measuring NLR in ICB for NSCLC provide compelling evidence in favor of a high NLR serving as a measure of adverse outcomes in the era of immunotherapy [Citation19,Citation30–32], which is consistent with our results. Notably, our cutoffs for some of the biomarkers studied here are in contrast to values used in recent ICB data in NSCLC. Some recent studies have used a reference cutoff of 5 for NLR [Citation19,Citation30,Citation32], which is based on previous historical standards derived from ICB in melanoma [Citation19,Citation33]. Conversely, recent studies from two European [Citation21,Citation25] and two American [Citation34,Citation35] groups used a different NLR sub-stratification. Despite the differences in the cutoffs, all these studies imply an elevated NLR to correlate with inferior survival. Given the variability in the cutoffs, serial monitoring of these biomarkers rather than an absolute value at a single time point may hold a higher degree of significance.
One of the objectives of this study was to assess the utility of the mGPS in the era of immunotherapy. Data in a recently published abstract from a French group have depicted mGPS to have a predictive value in NSCLC patients on nivolumab [Citation23]. However, our results did not denote a similar role for mGPS. Evaluating mGPS in a larger sample size of patients on ICB with a more extended follow-up may provide a better insight into the factors responsible for such differences.
With the increasing use of ICB, there are a distinct set of irAEs that have the potential to cause high morbidity and treatment discontinuations [Citation36]. Thus far, factors predisposing some patients to high grade or early irAEs remain unclear. We observed a direct relationship in the development of irAEs and a simultaneous rise in CRP compared to baseline levels (). This is analogous to the cytokine release storm (CRS) observed in adoptive T-cell therapy which is characterized by a similar CRP surge [Citation37]. This intriguing observation is hypothesis generating and raises the speculation of dysregulated immune activation in the context of ICB. We propose that similar to the CRS, immunological perturbations post-ICB likely result in a cytokine surge causing a shift towards a severe pro-inflammatory state. These pro-inflammatory alterations could potentially explain the development of the irAE. Although these observations are very preliminary, understanding these mechanisms could pave the way for treatment modalities explicitly targeting inflammatory cascades implicated in the pathophysiology irAEs.
There are several shortcomings of the study. First, the design in which the patients were recruited and the retrospective nature of the data analysis can be prone to bias. Due to of the lack of an appropriate comparator arm, the predictive or prognostic utility of these peripheral blood-based inflammatory markers is unclear at this point. Second, our results were derived from a cohort that was not chemotherapy naive at ICB initiation, which may have influenced the degree of baseline inflammation and hence the inflammatory biomarkers. As such, these results may not be entirely applicable in the setting of upfront ICB in NSCLC. Third, the cutoff for the biomarkers is a matter of debate as depicted from comparison with recently published data. The arbitrary nature of these variables precludes any practice changing conclusions and warrants further analysis. Lastly, it would be prudent to have a pooled multi-institutional analysis with an extended follow-up to have a clinically meaningful understanding of the utility of these biomarkers in survival stratification of patients on ICB.
Our study serves as a prelude to understanding the association of readily available inflammation linked peripheral blood-based biomarkers with survival in ICB. These biomarkers may serve as potential surrogates for the compound immune interactions taking place within the tumor microenvironment. We do understand that treating patients solely based on biochemical markers has several limitations and should not be the sole parameter for clinical decision-making. However, the use of peripheral blood-derived inflammatory biomarkers in survival stratification of ICB treated patients can be a viable option that could enable optimal designing of patient-tailored treatments and thus help guide immunotherapy.
Abdul_Rafeh_Naqash_et_al._Supplementary.docx
Download MS Word (17.9 KB)Acknowledgments
Consent for publication: Consent for publication and IRB approval have been obtained as part of the P-MAIT study at East Carolina University (UMCIRB-15-001400), that states that the patients have consented to the reporting of any medical/clinical/laboratory and radiological data generated as part of this IRB approved study.
Disclosure statement
The authors declare that they have no conflicts of interest relevant to this study.
AR Naqash, A Hegde, CR Cherry, GK Dy, M Muzaffar and M Hafiz do not report any disclosures.
CRG Stroud serves on the speaker's bureau for Genentech.
P Walker serves on the speaker's bureau for Genentech and Biodesix.
M.U. Butt is supported by the National Institute of Health Ruth. L Kirschtein national research service award (T32HL091812).
References
- Rafei H, El-BAahesh E, Finianos A, et al. Immune-based therapies for non-small cell lung cancer. AR. 2017;37:377–387.
- Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4.
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. JCO. 2015;33:1974–1982.
- Maleki Vareki S, Garrigós C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol/Hematol. 2017;116:116–124.
- Khunger M, Hernandez AV, Pasupuleti V, et al. Programmed cell death 1 (PD-1) ligand (PD-L1) expression in solid tumors as a predictive biomarker of benefit from PD-1/PD-L1 axis inhibitors: a systematic review and meta-analysis. JCO Precis Oncol. 2017;1. DOI:10.1200/PO.16.00030
- Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? J Immunother Cancer. 2016;4:48.
- Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856.
- Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14:433–439.
- Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–3355.
- Laird BJ, Kaasa S, McMillan DC, et al. Prognostic factors in patients with advanced cancer: a comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Cancer Res. 2013;19:5456–5464.
- McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540.
- Simmons CP, Koinis F, Fallon MT, et al. Prognosis in advanced lung cancer—a prospective study examining key clinicopathological factors. Lung Cancer. 2015;88:304–309.
- Mei Z, Shi L, Wang B, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13.
- Sun K, Chen S, Xu J, et al. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1537–1549.
- Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862.
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69.
- Lang T. Documenting research in scientific articles: guidelines for authors: 3. Reporting multivariate analyses. Chest. 2007;131:628–632.
- Shrotriya S, Walsh D, Bennani-Baiti N, et al. C-Reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS One. 2015;10:e0143080.
- Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7.
- Mezquita L, Charrier M, Auclin E, et al. Neutrophil-to-lymphocyte and other ratios as prognostic and predictive markers of immune checkpoint inhibitors in advanced NSCLC patients. J Thorac Oncol. 2017;12:S1315–S13S6.
- Russo A, Scimone A, Picciotto M, et al. Association between baseline absolute neutrophil count (ANC), derived neutrophil-to-lymphocyte ratio (dNLR), and platelet-to-lymphocyte ratio (PLR) and response to nivolumab (Nivo) in non-small cell lung cancer (NSCLC): a preliminary analysis. J Clin Oncol. 2017;35:e14617.
- Hiltermann TJN, Hijmering-Kappelle L, Groen HJM. Markers of response to PD-1 inhibition. J Clin Oncol. 2017;35:e14505.
- Gervais C, Boudou-Rouquette P, Jouinot A, et al. Predictive and prognostic value of systemic inflammatory response biomarkers in patients receiving nivolumab for metastatic non-small cell lung cancer (NSCLC). J Clin Oncol. 2017;35:3055.
- Brustugun OT, Sprauten M, Helland A. C-reactive protein (CRP) as a predictive marker for immunotherapy in lung cancer. J Clin Oncol. 2016;34:e20623.
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181.
- Canna K, McArdle PA, McMillan DC, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92:651–654.
- Simeone E, Gentilcore G, Giannarelli D, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother. 2014;63:675–683.
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–446.
- Muller I, Munder M, Kropf P, et al. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30:522–530.
- Preeshagul I, Sullivan K, Paul D, et al. P3.02c-078 the utilization of pre-treatment neutrophil to lymphocyte ratio as a predictive marker for efficacy of immunotherapy in non-small cell lung cancer. J Thorac Oncol. 2017;12:S1325.
- Tiseo M, Veneziani M, Gelsomino F, et al. Circulating immune-profile as predictor of outcome in advanced NSCLC patients treated with nivolumab. J Clin Oncol. 2017;35:e14514.
- Labomascus S, Fughhi I, Bonomi P, et al. Neutrophil to lymphocyte ratio as predictive of prolonged progression free survival (PFS) and overall survival (OS) in patients with metastatic non-small cell lung cancer (NSCLC) treated with second-line PD-1 immune checkpoint inhibitors. J Clin Oncol. 2017;35:e14530.
- Ferrucci PF, Gandini S, Battaglia A, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112:1904–1910.
- Soyano AE, Dholaria BR, Marin-Acevedo JA, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced lung cancer in patients treated with anti-PD-1 antibody. J Clin Oncol. 2017;35:e20599.
- Patil PD, Khunger M, Rakshit S, et al. Pre-treatment hematological markers as a predictive biomarker for survival in patients with non-small cell lung cancer treated with nivolumab. J Clin Oncol. 2017;35:11547.
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148.
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195.
