Abstract
Background: Biliary tract cancer (BTC) is an uncommon cancer with an unfavorable prognosis. Since 2010, the standard of care for patients with unresectable BTC is palliative treatment with gemcitabine plus cisplatin, based on the landmark phase III ABC-02 trial. This current study aims to evaluate the efficacy and safety of gemcitabine and cisplatin in patients with unresectable cholangiocarcinoma and gallbladder cancer in daily practice that meet the criteria for the ABC-02 trial in comparison to patients who did not.
Methods: Patients diagnosed with unresectable BTC between 2010 and 2015 with an indication for gemcitabine and cisplatin were included. We divided these patients into three groups: (I) patients who received chemotherapy and met the criteria of the ABC-02 trial, (II) patients who received chemotherapy and did not meet these criteria and (III) patients who had an indication for chemotherapy, but received best supportive care without chemotherapy. Primary outcome was overall survival (OS) and secondary outcome was progression-free survival (PFS).
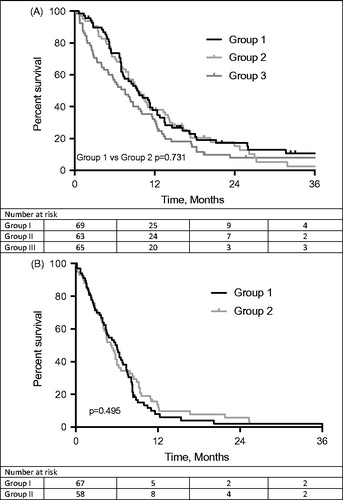
Results: We collected data of 208 patients, of which 138 (66.3%) patients received first line chemotherapy with gemcitabine and cisplatin. Median OS of 69 patients in group I, 63 patients in group II and 65 patients in group III was 9.6 months (95%CI = 6.7–12.5), 9.5 months (95%CI = 7.7–11.3) and 7.6 months (95%CI = 5.0–10.2), respectively. Median PFS was 6.0 months (95%CI = 4.4–7.6) in group I and 5.1 months (95%CI = 3.7–6.5) in group II. Toxicity and number of dose reductions (p = .974) were comparable between the two chemotherapy groups.
Conclusion: First-line gemcitabine and cisplatin is an effective and safe treatment for patients with unresectable BTC who do not meet the eligibility criteria for the ABC-02 trial. Median OS, PFS and treatment side effects were comparable between the patients who received chemotherapy (group I vs. group II).
Introduction
Biliary tract cancer (BTC) is an uncommon cancer in developed countries consisting of cholangiocarcinoma and gall bladder cancer. The incidence of gall bladder cancer and extrahepatic cholangiocarcinoma in the European Union is 3.2 and 5.4/100,000 per year for males and females, respectively [Citation1]. There are approximately 600 new cases of BTC in the Netherlands per year, and the incidence is rising [Citation2]. Surgical resection is the only curative treatment for patients with BTC but most patients have (locally) advanced disease or metastasis at presentation and are not eligible for surgical resection [Citation3,Citation4].
Patients with unresectable BTC are currently treated with a combination of gemcitabine and cisplatin, based on the phase III ABC-02 trial. This trial demonstrated a significant survival advantage of this combination without the addition of substantial toxicity compared to gemcitabine monotherapy [Citation5]. The promising results of this clinical trial led to incorporating this treatment regimen, consisting of 1000mg/m2 gemcitabine and 25mg/m2 cisplatin in a three-weekly cycle with administrations on day one and eight, in national and international guidelines, including the European Society for Medical Oncology (ESMO) guideline [Citation6].
Although this combination of gemcitabine plus cisplatin showed survival advantage when compared with gemcitabine alone, this regimen was studied in a group of patients complying with the inclusion criteria of the ABC-02 trial. However, most patients in clinical practice do not fulfill these criteria and the efficacy and toxicity of this regimen has not been evaluated in these patients. Furthermore, the effect of gemcitabine plus cisplatin in patients with unresectable BTC has not been compared with patients receiving best supportive care.
No difference in median overall survival (OS) between trial and non-trial patients was observed in similar retrospective studies in colorectal and breast cancer [Citation7,Citation8]. In a study performed in men with metastatic castration resistant prostate cancer, it has been demonstrated that treatment in daily practice is associated with a shorter survival and more toxicity compared with men treated in a clinical trial [Citation9].
Because of the poor prognosis of patients with BTC and the possible adverse effects of chemotherapy, it is important to know if this ABC-02 chemotherapy treatment regimen could also be used in patients who do not fulfill the original inclusion criteria [Citation5]. Therefore the aim of this study was to evaluate the efficacy and safety of gemcitabine and cisplatin in daily practice in unresectable BTC patients, who do not meet the eligibility criteria for the ABC-02 trial, compared with those who meet the inclusion criteria for this trial.
To be able to answer this question, it is necessary to study a subsequent population of patients that is treated in daily practice with a combination of gemcitabine and cisplatin. In addition, we aimed to compare patients who received chemotherapy and patients who received best supportive care.
Methods
Study population and data acquisition
All patients with unresectable BTC between January 2010 and January 2015 in the Academic Medical Center (AMC), Amsterdam, the Netherlands and Erasmus MC University Medical Center (EMC), Rotterdam, the Netherlands, were identified. Referring hospitals were contacted for additional data on the referred BTC patients. BTC was defined as intra- or extrahepatic cholangiocarcinoma or gallbladder cancer. If no histopathological evidence was obtained, the diagnosis was established by the multidisciplinary hepatopancreatobiliary team based on clinical, radiological, endoscopic, laboratory findings and follow-up. Patients were deemed unresectable when distant metastases were present or when radical resection was not possible due to locally advanced disease. Also recurrent disease after surgery was considered as unresectable. Patients were excluded if they received first-line chemotherapy other than gemcitabine and cisplatin. Patient characteristics (e.g., age, sex), clinical parameters (e.g., cholangitis), laboratory results (e.g., white-cell count, platelet count), chemotherapy treatment details (e.g., dose, number of cycles, toxicity) and previous interventions (surgery, percutaneous transhepatic drainage (PTC), endoscopic retrograde cholangiopancreatography (ERCP), radiotherapy) were collected from medical records.
The study population was divided into three groups: (I) patients who received chemotherapy and met the criteria of the ABC-02 trial, (II) patients who received chemotherapy and did not meet these criteria and (III) patients who were eligible for chemotherapy but received best supportive care without any chemotherapy. The criteria used in the ABC-02 trial [Citation5] are: histopathological or cytologic confirmation, an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2, a total serum bilirubin level of 1.5 times the upper limit of normal (ULN) range or less, liver-enzyme levels of 5 times the ULN range or less, levels of serum urea and serum creatinine of 1.5 times the ULN range or less and a calculated glomerular filtration of 45ml/min or higher. The Institutional Review Boards of both centers approved the study and the need for informed consent was waived. Toxicity was scored using the common terminology criteria for adverse events (CTCAE) version 4.03 [Citation10]. Radiologic response evaluation was assessed using response evaluation criteria in solid tumors (RECIST) [Citation11].
Study objectives
The primary objective was to compare OS in patients treated with gemcitabine plus cisplatin who do versus who do not fulfill the inclusion criteria of the ABC-02 trial. Secondary objectives were to investigate differences in progression-free survival (PFS) and toxicity between the two predefined groups. We also compared these data with the patients that were eligible for treatment but opted for best supportive care instead of chemotherapy (group III).
Statistical analysis
All statistical analyses were performed using SPSS version 22 (IBM corp). Descriptive statistics for categorical variables were reported as percentages, and continuous variables were reported as medians and ranges. Categorical variables were compared using Chi square test and continuous variables were compared using the independent-samples t-test.
Dose intensity was calculated as the cumulative dose of gemcitabine per body surface area divided by the time between the date of the first administration and the end of the last cycle of chemotherapy. The same calculation was used to calculate dose intensity of cisplatin. The dose intensity of 100% gemcitabine was defined as 666.7 mg/m2/week and for cisplatin as 16.7mg/m2/week [Citation6].
The OS of BTC patients who received chemotherapy was calculated from the date of first gemcitabine and cisplatin administration. Survival of the patients who did not receive chemotherapy was calculated from the date of initial diagnosis to death with censoring for patients alive at the last moment of follow-up. An additional calculation was performed where OS of BTC patients who received chemotherapy was calculated from the date of initial diagnosis to be able to compare this to patients who did not receive chemotherapy. The PFS in patients receiving chemotherapy was calculated from the date of first gemcitabine and cisplatin administration to the date on which radiological or clinical progression was determined with censoring for patients with no progressive disease at the end of follow-up (1 July 2015). The Kaplan–Meier method was used to estimate OS. Multivariate analysis was performed with Cox Proportional Hazards Model. Survival status was updated using the municipal records database on 10 February 2016.
Results
Patients and treatment
In total, 208 patients with unresectable BTC were identified and formed the study cohort (). The majority of our cohort received gemcitabine and cisplatin treatment (n = 138, 66.3%). Of these, 74 (53.6%) patients received chemotherapy and met the criteria of the ABC-02 trial (group I) and 64 (46.4%) patients received chemotherapy and did not meet these criteria (group II; see ). Seventy patients were eligible for chemotherapy, but received best supportive care without any chemotherapy (group III). Patient’s choice was the most frequent reason for not receiving chemotherapy. presents the baseline patient characteristics. Most patients were male (54.3%) and the median age of patients who received chemotherapy and the patients who received best supportive care were 63 and 72 years, respectively.
Figure 1. Flow chart. ErasmusMC, Erasmus Medical Center. AMC, Amsterdam Medical Center. The ErasmusMC cohort consists of only perihilar cholangiocarcinoma patients and some additional BTC patients from a systematic search in the pharmacy registers. The AMC cohort consists of all unresectable BTC patients between January 2010 and January 2015. BTC was defined as intra- or extrahepatic cholangiocarcinoma or gallbladder cancer.
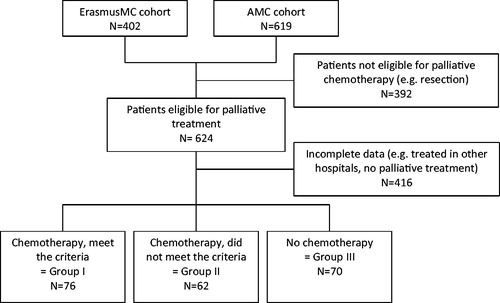
Table 1. Did not meet the criteria of the ABC-02 trial based on.
Table 2. Patients characteristics.
Group I had more patients with gallbladder tumors and less patients bile duct tumors compared to group II (gallbladder: 23.0% vs. 12.5%; bile duct: 64.9% vs. 81.3% p = .303). In group II, significantly more patients had interventions prior to chemotherapy, most frequently a PTC (6.8% vs. 26.6% p = .002). The majority of patients in group III had bile duct as primary tumor site (67.6%) and 91.2% of group III patients underwent a previous therapy.
Overall survival and progression free survival
The median OS of the entire cohort was 8.8 (95%CI = 7.5–10.1) months. Patients who received chemotherapy and met the criteria of the ABC-02 trial had a median OS, calculated from date of first administration of chemotherapy, of 9.6 (95%CI = 6.7–12.5) months, which was comparable with 9.5 (95%CI = 7.7–11.3) months in patients who received chemotherapy and did not meet these criteria (p = .731; ). If OS is calculated from date of initial diagnosis, all patients treated with gemcitabine plus cisplatin (group I and II combined) had a median OS of 14.8 (95%CI = 11.7–17.8) months compared with patients who received best supportive care without chemotherapy of 7.6 (95%CI = 5.0–10.2) months (p < .001). Median PFS of patients in group I was 6.0 (95%CI = 4.4–7.6) months, compared to 5.1 (95%CI = 3.7–6.5) months of patients in group II (p = .495; ). In a multivariable analysis that included WHO performance status, body mass index (BMI), extent of disease, primary tumor site and previous therapies, we did not identify receiving chemotherapy according to the ABC-02 criteria or not as an independent prognostic factor for survival (HR = 0.83, 95%CI = 0.56–1.24).
Chemotherapy treatment
shows the comparison of outcomes of the patients who received chemotherapy and met the criteria of the ABC-02 trial versus those who received chemotherapy and did not meet these criteria. The dose-intensity is comparable between both groups. The main reason for ceasing chemotherapy treatment was disease progression.
Table 3. Treatment and outcomes.
Toxicity
shows the grade 3 and 4 toxicity of the chemotherapy treatment in group I and II. Patients in group II more often had a decrease in platelet count as a result of chemotherapy treatment (8.2% vs. 18.8%, p=.079). Other toxicities were comparable between the two groups.
Table 4. Grade 3 or 4 toxic effects during treatment.
Discussion
In this study, we found that patients receiving treatment with gemcitabine and cisplatin who did not meet the inclusion criteria of the ABC-02 trial, have comparable OS, PFS, toxicity and chemotherapy dose reduction rates compared with patients who fulfilled the inclusion criteria of the ABC-02 trial.
Biliary tract cancer (BTC) is an uncommon cancer with a poor prognosis. The ABC-02 trial demonstrated that a chemotherapy combination of gemcitabine plus cisplatin was associated with a significant survival advantage without the addition of substantial toxicity compared to gemcitabine monotherapy. However, inclusion criteria are strict and most patients in clinical practice do not fulfill the inclusion criteria of this ABC-02 trial. To provide more evidence about the efficacy and safety of chemotherapy in the heterogeneous group of unresectable BTC patients, it is necessary to extend inclusion criteria [Citation12].
To our best knowledge, this is the largest retrospective analysis since gemcitabine and cisplatin has become the standard chemotherapeutic regimen for advanced BTC. One of the strengths of this study is the large patient population with BTC derived from two specialized centers, including detailed data on the diagnosis, treatment, toxicity and tumor evaluation. All patients received the same standard treatment according to the ESMO guidelines [Citation6]. Not all of these patients are treated in the specialized centers, but were referred back to peripheral hospitals to receive the chemotherapy.
When comparing our study cohort with the ABC-02 trial cohort, there are several differences to note. Considering patients’ characteristics, the primary tumor site in patients treated in the ABC-02 trial was more often gallbladder and less often bile duct (gallbladder: 18.1% vs. 35.8%; bile duct: 72.5% vs. 59.8%). The median OS in patients receiving chemotherapy in our study cohort was 9.5 (95%CI = 7.9–11.1) months, compared with a median OS of 11.7 (95%CI=-9.5–14.3) months in the ABC-02 trial. Median PFS of patients treated in daily practice was 5.6 (95%CI = 4.5–6.7) months compared with 8.0 (95%CI = 6.6–8.6) months in the ABC-02 trial.
The different survival between the patients in our study cohort and the ABC-02 trial can be explained by the differences in the patients’ characteristics such as primary tumor site and previous therapies. More previous therapies might lead to a selection bias, because patients might have a positive effect of these other therapies besides the chemotherapy. In the ABC-02 trial, more patients had gallbladder cancer in comparison to our study cohort. As seen in the ABC-02 trial, there was a higher partial response rate to chemotherapy in patients with gallbladder cancer compared to patients with cholangiocarcinoma. In general, patients with gallbladder cancer have a shorter OS compared to cholangiocarcinoma, but this might be different in patients who are treated with gemcitabine and cisplatin [Citation13]. The way OS is calculated, can also contribute to a difference in OS between our cohort and the OS in patients in the ABC-02 trial. In the ABC-02 trial, OS is calculated from date of randomisation in comparison with date of first administration of chemotherapy in our study cohort.
In our study cohort, we also observed a higher toxicity rate than in the ABC-02 trial. Neutropenia and thrombocytopenia occurred more frequently in our study. Since this is a retrospective analysis, not all toxicities were reported systematically, which may have led to an underestimation of toxicity. The higher toxicity rate in our study, resulting in more dose reductions, might be a possible explanation for the lower median survival in patients treated in daily practice. Moreover, the higher toxicity rate in our study cohort can be explained by less restrictive inclusion criteria for part of the patients in comparison to the ABC-02 trial.
The AMC and EMC are highly specialized tertiary institutes for BTC, which may have caused a selection bias, because the majority of these referred patients needed drainage and may possibly have had a poorer baseline situation than patients who are not referred to the AMC or EMC. This could explain the difference in median OS and PFS between patients who received chemotherapy in daily practice and patients in the ABC-02 trial (OS 9.5 vs. 11.7 months; PFS 5.6 vs. 8.0 months), because patients without drainage problems are more likely not to be referred to a highly specialized tertiary institute.
In contrast with a recent similar retrospective analysis in 26 metastatic BTC patients [Citation14], we observed a difference in OS in patients treated with gemcitabine and cisplatin in daily practice compared with patients in the ABC-02 trial. Almost half of these patients [Citation14] (49%) received second or third line chemotherapy, which may explain the higher OS in the other retrospective analysis (9.5 vs. 10.5 months).
When comparing patients who received chemotherapy in daily practice and met the criteria of the ABC-02 trial and patients who did not meet these criteria, we found no differences in median OS or PFS. Patients in group I had a higher 2.5-year survival rate in comparison with patients in group II (2.5-year survival: 12.9% vs. 5.1% respectively). This suggests a treatment advantage of chemotherapy, after careful patient selection, based on criteria used in the original clinical trials.
Although eligible for chemotherapy treatment, 70 patients received best supportive care rather than chemotherapy. These patients were older than patients who did receive chemotherapy. Although, the ECOG performance status was missing in 32.9% (n = 23) of patients that received best supportive care, the impaired clinical condition of these patients may explain why they did not receive any chemotherapy but best supportive care instead.
Several limitations of the current study should be mentioned. Since it is a retrospective analysis, not all required data were systematically reported and therefore not available. The administration of chemotherapy and toxicities were not reported unambiguously.
In conclusion, our study shows that patients who did not meet the inclusion criteria of the ABC-02 trial but receiving an identical chemotherapy regimen, had comparable OS, PFS, toxicity and chemotherapy dose reduction rates compared with patients who did fulfill the ABC-02 trial inclusion criteria. Patients with unresectable BTC who received gemcitabine plus cisplatin had a better OS than patients who received best supportive care in real life practice. Patients with unresectable BTC who do not meet the original inclusion criteria used in the ABC-02 trial should still be considered for gemcitabine plus cisplatin treatment.
Disclosure statement
The authors have declared no conflicts of interest.
Funding
No funding was received.
References
- Cidon EU. Resectable cholangiocarcinoma: reviewing the role of adjuvant strategies. 2016;10:43–48.
- Intergraal Kankercentrum Nederland [Internet]. 2015 [cited 2015 Jun 15]. Available from: www.cijfersoverkanker.nl.
- Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517.
- Hartog H, Ijzermans JNM, van Gulik TM, et al. Resection of perihilar cholangiocarcinoma. Surg Clin North Am. 2016;96:247–267.
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281.
- Valle JW, Borbath I, Khan S. a, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28–v37.
- Mol L, Koopman M, van Gils CWM, et al. Comparison of treatment outcome in metastatic colorectal cancer patients included in a clinical trial versus daily practice in The Netherlands. Acta Oncol. 2013;52:950–955.
- Gallagher CM, More K, Masaquel A, et al. Survival in patients with non-metastatic breast cancer treated with adjuvant trastuzumab in clinical practice. Springerplus. 2016;5:395.
- Templeton AJ, Vera-Badillo FE, Wang L, et al. Translating clinical trials to clinical practice: outcomes of men with metastatic castration resistant prostate cancer treated with docetaxel and prednisone in and out of clinical trials. Ann Oncol. 2013;24:2972–2977.
- Common terminology criteria for adverse events v4.0 (CTCAE). Available from: http://www.cancer.gov
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247.
- Park JO, Oh D-Y, Hsu C, et al. Gemcitabine plus cisplatin for advanced biliary tract cancer: a systematic review. Cancer Res Treat. 2015;47:343–361.
- Gallardo J, Betzabe R, Villanueva L, et al. Gallbladder cancer, a different disease that needs individual trials. J Clin Oncol. 2005;23:7753–7754.
- Agarwal R, Sendilnathan A, Siddiqi NI, et al. Advanced biliary tract cancer: clinical outcomes with ABC-02 regimen and analysis of prognostic factors in a tertiary care center in the United States. J Gastrointest Oncol. 2016;6:996–1003.