Abstract
Background: Most studies from high income countries consistently report that preexisting diabetes reduces overall survival of cancer patients. We examined this association in a retrospective cohort study using two nation-wide population-based data sets in Latvia.
Material and methods: The Cancer Register, linked with the Diabetes Register and Causes of Death Database, was the first data source used to select 22,936 men and 25,338 women with cancer diagnosed from 2009 to 2013. The follow-up period ended on 28 February 2015. The National Health Service data served as a second data source, which was used to select 10,130 men and 13,236 women with cancer as the main diagnosis, who were discharged from oncology hospitals from 2009 to 2012. Prescriptions of reimbursed antidiabetic medications indicated prior diabetes status. The follow-up period started at the date of discharge and ended on 31 December 2013. A Cox proportional hazards model was used to assess association between preexisting diabetes and all-cause mortality, adjusted for age.
Results: Men with preexisting diabetes had better overall short-term survival: the age-adjusted hazard ratios (95% CI) were 0.86 (0.79–0.93) for the first year and 0.89 (0.80–0.98) for the first two years after cancer diagnosis according to the disease register and health service data, respectively. After three full follow-up years, their relative mortality increased, with an age-adjusted hazard ratio of 1.60 (1.28–1.99). Among women, preexisting diabetes was associated with slightly higher all-cause mortality during the entire follow-up period, with age-adjusted hazard ratios of 1.17 (1.10–1.24) for the disease register data and 1.11 (1.02–1.21) for the health service data.
Conclusion: Interestingly, we found better overall survival of diabetic men during the first years after cancer diagnosis. We hypothesize that access to health services may be advantageous to diabetic patients who are in close contact with the healthcare system.
Background
Diabetes mellitus and cancer are two common diseases affecting ageing populations worldwide. Furthermore, the combination of both conditions is not rare. Using modeled age-specific transition rates from healthy status to diabetes, cancer or death, it has been estimated, according to different scenarios, that the life-time risk of developing both diseases in the Danish population is 15% for men and 13% for women and among individuals with diabetes, 43% of men and 41% of women will also be diagnosed with cancer [Citation1]. Treatment of cancer can be challenging in patients with any prior co-morbidity, including diabetes. Several reviews and meta-analyses agree that preexisting diabetes increases all-cause mortality in cancer patients [Citation2–10]. The effect size differs by cancer site, but for all cancer types, mortality is increased by approximately 40% [Citation2]. A review by Renehan et al. [Citation7] summarizes not only the methodological issues but also the mechanisms of this association; potential explanations range from inequalities in screening uptake to adverse effects of cancer therapies and tumor biology. However, most studies have been conducted in Western high-income countries with well-functioning health systems. Latvia, the former republic of the Soviet Union, together with other countries in Central and Eastern Europe, differ from Western European countries in terms of the population health status and risk factor distribution [Citation11]. Life expectancy in Latvia is still among the lowest in the European Union. In 2015, life expectancy at birth was 69.7 years for males and 79.5 years for females; of those years, 51.8 and 54.1, respectively, were healthy life years [Citation12].
The diabetes prevalence is moderate in Latvia compared with other developed countries. By the end of 2015, there were 86,941 diabetes patients according to the Diabetes Register of Latvia, corresponding to a diabetes prevalence of 4.4% [Citation13]. Overall cancer incidence in Latvia is comparable with that in other countries, particularly Eastern Europe. The age-standardized (world standard) incidence rate (SIR) for all sites from 2003–2009 was 316.8 per 100,000 men and 214.7 per 100,000 women, with lung cancer being the most common cancer among men (SIR 63.4 per 100,000) and breast cancer being the most common among women (SIR 48.4 per 100,000) [Citation14]. Cancer mortality is comparatively high in Latvia, even for Eastern Europe. For example, the age-standardized (world standard) death rate from all cancers in 2009–2013 was 195.6 per 100,000 men and 97.4 per 100,000 women [Citation15].
Although there are some differences in morbidity and mortality indicators, it is likely that diabetes negatively affects cancer patients prospects in any population. Thus, we hypothesize that diabetes will have an adverse effect on cancer patient survival in a Latvian population. The aim of the study was to assess the association between preexisting diabetes mellitus and overall survival of adult cancer patients in Latvia.
Material and methods
A retrospective cohort study was conducted using two different data sets: first, population-based disease registers, namely, the Cancer Register, Diabetes Register and Causes of Death Database and second, health-care service data, which are administrative data. The health-care service data were chosen as an alternative data source because of a potential issue with validity in the disease register data related to the diabetes patient registration procedure (discussed below). Both data sets partially overlapped in the covered cancer patients and the linkage between them would be an optimal approach but was impossible because of different data owners. The system of disease registers is owned by the Centre for Disease Prevention and Control (CDPC) of Latvia, whereas the owner of the health service data is the National Health Service (NHS) of Latvia. The current legislation in Latvia does not provide a clear procedure for exchange of personal data between different institutions for research purposes. Therefore, individual-level data linkage to create both data sets was performed by data owners inside each institution and anonymized data were released to the researchers. Linkage was based on unique personal identification numbers, which are used throughout all information systems in Latvia and should match the individual’s information in the Population Register, such as the name, sex, date of birth and if applicable, death. The study received approval from the Riga Stradins University Ethics Committee.
Disease register data
Data source description and quality
The Cancer Register and the Diabetes Register are population-based databases and cover all residents in Latvia. The Cancer Register was officially established in 1993 and the reporting of incident cancer cases is mandatory; it also includes retrospective registration, e.g., after death. Although some underreporting of mainly ambulatory-treated cancers, such as skin cancers and melanomas, is suspected, the Cancer Register complies with international standards of cancer surveillance [Citation14] and its quality with regard to completeness and validity of case ascertainment has been rated sufficient to include Latvian data in such international projects as the EUROCARE-5 [Citation16]. Since September 2014, closer monitoring of cancer patients has been enforced and also requires follow-up reports about treatment changes, as well as recurrences and metastases. However, in the previous period, the focus was on complete identification of primary incident cancers, and the follow-up of existing cases was mainly limited to tracking deaths. The registration form does not include socio-economic status of the patient and information about possible risk factors, such as smoking or alcohol use, albeit included, is often missing. Recording of cancer stage, in contrast, has been always controlled by the Register staff; stage distribution according to the 0–IV staging system is regularly published in health statistics reports of the CDPC. Meanwhile, the quality of the detailed staging data (TNM, cancer specific), although required in the registration form, has not been rated sufficient for inclusion in data analysis by the EUROCARE-5 study group [Citation17].
The Diabetes Register was established in 1997. According to regulations, family doctors must annually report newly diagnosed diabetes patients (except for those with gestational diabetes) and provide updated information on previous cases. Although formal validation studies have not been performed, since 2007, completeness of the Diabetes Register is regularly examined by comparing records with the database of reimbursed medication prescriptions and prescribing physicians are actively questioned if unregistered patients are found. As it is primarily focused on clinical parameters, the registration form includes neither socio-economic nor lifestyle factors. Thus, more detailed analysis on pathways linking preexisting diabetes and cancer patient survival was not performed.
Every day at midnight, the vital status of patients registered in any of the disease registers is updated from the Causes of Death Database; the date of death can be considered accurate. The 10th Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) is used in all three aforementioned databases.
Study population
A flowchart () shows the selection procedure of the study population. From 1 January 2009 to 31 December 2013, patients with incident primary cancers (ICD-10 codes C00–C97) who were never registered in the register before were selected from the Cancer Register. After exclusion of 52 patients because of erroneous date of diagnosis, the 4712 cancer patients who were registered in the Diabetes Register (ICD-10 codes E10–E14) before the calendar year of cancer diagnosis were classified as having preexisting diabetes. A limitation of the Diabetes Register is that all records, including those of newly reported patients, have a date of reporting, not the date of diagnosis. Because most physicians submit their reports towards the end of the calendar year, in those cases where diabetes and cancer occurred in the same calendar year, the lack of a date of diabetes diagnosis challenged ascertainment of the actual temporal sequence of both diseases. Therefore, we excluded these 543 cases from analysis. The remaining 47,412 cancer patients were classified as non-diabetic individuals.
Figure 1. Flowchart of cancer patients by type of data source. (a) Population-based registers. (b)National Health Service.
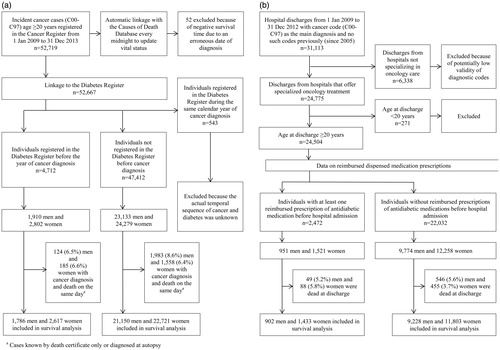
In total, there were 25,043 men with incident cancer, and 1910 (7.6%) of them had been registered in the Diabetes Register before the year of cancer diagnosis. Among 27,081 women with cancer, 2802 (10.4%) had prior diabetes. For 3850 cancer patients, the date of cancer diagnosis coincided with the date of death (cancer known only by death certificate or discovered at autopsy); therefore, these patients were not eligible for survival analysis and were excluded. The follow-up started on the date of cancer diagnosis and ended on 28 February 2015.
Health service data
Data source description and quality
The data owner NHS is responsible for contracting health-care providers and manages payments for publicly funded services. In 2005, the Management Information System was set up to administer all payments. Records of reimbursement claims include diagnostic codes (according to the ICD-10) of the main condition as well as comorbidities, if applicable. All residents are entitled to state-funded health care and the list of recipients is regularly updated through the link with the Population Register; thus, the date of each person’s death also appears in the records and must be accurate. Neither the cause of death nor cancer stage is available. The usage of private healthcare and out-of-pocket payments are not represented in the NHS data; however, treatments for cancer and diabetes are covered by the state budget. A limitation of health service data is the questionable validity of diagnostic codes, e.g., suspected conditions may not be distinguishable from confirmed diagnoses. The accuracy of diagnostic codes has never been formally examined. To ensure that the selected patients were indeed diagnosed with cancer, we were advised to use only hospital discharge data, not ambulatory care data and moreover, to consider only discharges from hospitals that provide specialized oncology treatment services.
The use of antidiabetic medications was used to identify diabetes patients. Data on reimbursed dispensed medication prescriptions in the Management Information System is available from 2006. In Latvia, all registered antidiabetic medications are reimbursed (more expensive drugs may require specific indications and assignment from an endocrinologist). As the medication reimbursement system is closely monitored by authorities (e.g., subsequent inquiries from the Diabetes Register), we considered the prescriptions to be a strong proxy indicator for diabetes.
Study population
The selection of the study population is presented in . During the period of 1 January 2009 to 31 December 2012, there were 31,113 individuals discharged from all hospitals with cancer codes C00–C97 as the main diagnoses, with no records of such codes previously (since 2005); these cases were presumed to be incident cancer cases. According to the Cancer Register, there were 44,824 incident cancer cases in Latvia during the same time period [Citation13]; thus, the total hospital discharge data corresponded to 69.4% of all cancer cases. Further restriction to the hospitals that offer specialized oncology treatment resulted in a selection of 24,504 individuals who were at least 20 years old.
After addition of reimbursed medication prescription data, 2472 cancer patients who had at least one prescription of antidiabetic medication dispensed before hospital admission were classified as diabetes patients. Of 10,725 men and 13,779 women with cancer diagnosis at discharge from hospitals providing specialized oncology treatment services, 951 (8.9%) and 1521 (11.0%), respectively, had preexisting diabetes. Those who died in the hospital were not eligible for inclusion in the survival analysis and were excluded. The rest were followed-up from the date of hospital discharge until death or 31 December 2013. The final number of included cancer patients and number of deaths during follow-up by the data source, sex and diabetes status are presented in .
Table 1. Characteristics of Latvian cancer patients included in the follow-up according to the data source.
Statistical analysis
Computer packages SPSS (version 22.0 for Windows; Armonk, NY, USA: IBM Corp.) and Stata (Release 11; College Station, TX, USA: StataCorp LP.) were used. Analysis was stratified by sex. In addition to all cancer types combined, site-specific cancers were analyzed separately if there were at least 50 observations in a subgroup. The mean value and standard deviation (SD) were estimated to summarize patient age, whereas the median value and interquartile range (IQR) were estimated to characterize follow-up time (). Subgroups were compared using the two-sample t-test, Mann–Whitney U test or χ2 test (and Fisher’s exact test if applicable). A Cox proportional hazards model was used to assess the association between prior diabetes and all-cause mortality, adjusted for age at cancer diagnosis or hospital discharge. The proportionality of hazards was evaluated by using log–log survival curves and follow-up time was split if the assumption was violated. A stratified Cox model was used to control for cancer stage (I, II, III, IV or missing) for the disease register data. Hazard ratios (HR) with 95% confidence intervals (CI) and p values are reported.
Results
presents HR estimates showing the all-cause mortality risk of diabetic cancer patients relative to non-diabetic cancer patients in both data sets. Overall, in both men and women, cancer patients with preexisting diabetes were older than non-diabetic cancer patients regardless of the data source (, p < .001). Therefore, age-adjusted HR estimates are discussed in the text, whereas unadjusted estimates are also available in . In both disease register data and health service data, there were no differences in follow-up time () between diabetic and non-diabetic men (p = .455 and .897, respectively), but among women, the median follow-up time was longer in non-diabetic than diabetic cancer patients (p < .001).
Table 2. Relative mortality of cancer patients with preexisting diabetes compared to non-diabetic cancer patients according to the data source.
Disease register data
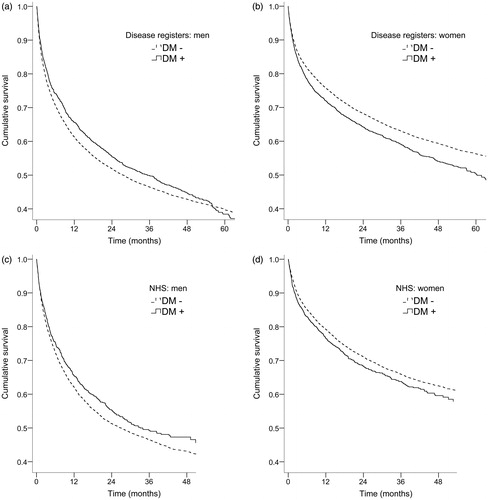
Among men, the association between preexisting diabetes and mortality varied with follow-up time (, ). During the first year after cancer diagnosis, cancer patients with diabetes experienced lower mortality than their non-diabetic counterparts (age-adjusted HR (aHR) 0.86; 95% CI 0.79–0.93). This mortality difference declined over the next two years, but after the third year of follow-up, diabetes was associated with 60% higher risk of death (aHR 1.60; 95% CI 1.28–1.99). Among women (), patients with diabetes had a slightly higher risk of death throughout the entire follow-up period (aHR 1.17; 95% CI 1.10–1.24).
Figure 2. Age-adjusted survival curves for cancer patients with and without preexisting diabetes by data source and sex. (a) Men, Latvian Registers of Cancer and Diabetes. (b) Women, Latvian Registers of Cancer and Diabetes. (c) Men, the National Health Service and (d) Women, the National Health Service.
Regarding site-specific cancers (, Supplemental Material), among men, prostate cancer patients with diabetes had higher mortality, but only after the full three-year follow-up period (aHR 1.92; 95% CI 1.28–2.88) and kidney cancer patients with diabetes had higher mortality only after the first year of follow-up (aHR 1.72; 95% CI 11.1–2.67). However, among men with bladder cancer, diabetes was associated with lower mortality during the first follow-up year (aHR 0.58; 95% CI 0.37–0.90). Among women, those with preexisting diabetes experienced higher mortality than non-diabetic women in the case of colorectal (aHR 1.17; 95% CI 1.00–1.36) or pancreatic (aHR 1.24; 95% CI 1.03–1.49) cancer and after the first follow-up year, those with uterine cancer and diabetes also exhibited increased mortality (aHR 1.38; 95% CI 1.03–1.86). Most associations between preexisting diabetes and survival were either non-existent or statistically non-significant (as illustrated by wide 95% CIs).
There were no significant differences in cancer stage distribution (), except for kidney cancer in men; a higher proportion of diabetic than non-diabetic men had a missing cancer stage (23.5 vs. 15.4%; p = .023). Further adjustment for cancer stage did not substantially affect the estimated associations (); only in men with stomach cancer, after one year of follow-up, the association between diabetes and increased risk of death became statistically significant (age and stage adjusted HR 1.67; 95% CI 1.05–2.66)
Table 3. Cancer stage distribution among patients with and without preexisting diabetes by cancer type according to the Latvian Registers of Cancer and DiabetesTable Footnotea.
Health service data
When all cancer types were combined, diabetic men (, ) had lower mortality than non-diabetic cancer patients during the first two years after hospital discharge and the age-adjusted HR was 0.89 (95% CI 0.80–0.98). After the first two follow-up years, diabetes was not associated with mortality (aHR 1.03; 95% CI 0.74–1.43). Among women (), diabetes increased the probability of death by 11% (aHR 1.11; 95% CI 1.02–1.21). Regarding specific cancer sites (, Supplemental Material), preexisting diabetes was associated with lower mortality among men with bladder cancer (aHR 0.45; 95% CI 0.27–0.76) during the entire follow-up period. Among women with stomach cancer, prior diabetes was associated with an approximately two-fold increase in mortality compared with non-diabetic individuals (aHR 2.09; 95% CI 1.20–3.64), but only after the first follow-up year. Pre-existing diabetes also increased the risk of death in women with ovarian cancer (aHR 1.42; 95% CI 1.02–1.99). Some associations suggesting either higher (e.g., in men with kidney cancer) or lower (e.g., in women with stomach cancer, during the first follow-up year) mortality risk in cancer patients with diabetes were non-significant, but for many cancer sites diabetes was not associated with survival.
Discussion
The aim of our study was to assess the impact of preexisting diabetes mellitus on cancer patient overall survival in the Latvian population. Two relatively independent data sources were used to improve the validity of the findings: population-based disease registers, namely, the Cancer Register and Diabetes Register and data on reimbursed health services. Although both data sources cover the entire Latvian population, in the case of health service data, the decision to opt for higher validity of cancer diagnoses and therefore, to include only discharges from hospitals with specialized oncology treatment services led to a selection of patients being introduced. Consequently, the results for both data sets were not identical but were similar when all cancer types were combined. In our study, preexisting diabetes, adjusted for age differences, was associated with a slight (11–17%) increase in all-cause mortality among women. Diabetic men, in turn, had 10–15% lower mortality than non-diabetic individuals during the first one to two years after cancer diagnosis; however, their relative mortality increased (60% higher) in the long term after three full follow-up years (according to the disease register data). With regard to specific cancer sites, the strength and direction of the association were less consistent between the two data sets, which may be explained by the different profiles of patients admitted to specialized hospitals. In addition, the precision of estimates may have suffered due to the small subgroup sizes.
The published literature concludes that the all-cause mortality risk is higher in cancer patients with diabetes [Citation2–10]. Apart from several plausible explanations related to differences in cancer detection, treatment choices or side effects [Citation7,Citation10], diabetes may affect overall survival independently of cancer progression, as it is a well-known risk factor for cardiovascular diseases [Citation18]. Indeed, evidence that cancer-specific survival is affected by diabetes is less consistent [Citation2,Citation4,Citation6,Citation8–10], suggesting that non-cancer mortality might contribute to the poorer prognosis.
Thus, the association between preexisting diabetes and all-cause mortality of cancer patients in our study showed a different pattern, particularly among men. First, the seemingly protective effect of diabetes among male cancer patients contradicts existing evidence; second, the direction of the association changed during the follow-up period: after three or more years of cancer diagnosis, diabetes appeared to be detrimental to survival. Regarding the latter, previous studies have shown varying effects of diabetes with follow-up times. Both Ranc et al. in Danish [Citation19] and Walker et al. in Scottish [Citation20] population-based register studies observed a weaker association during the first years of follow-up (in fact, the association was absent in Scottish patients with colorectal cancer) that became stronger with increasing follow-up time. Such an observation conforms with the interpretation that causes unrelated to cancer are responsible for the mortality increase in diabetes patients compared with non-diabetes patients [Citation20].
Regarding the improved overall survival of diabetic men during the first years after cancer diagnosis, both biases and local circumstances should be considered. The disease register data may be affected by registration procedures for the Diabetes Register. All reports, including those for newly diagnosed patients, must be submitted annually, although not necessarily at the time of diagnosis. In practice, the majority of reports are received towards the end of the calendar year. As the main purpose of the Diabetes Register is to monitor the quality of diabetes care, greater efforts are dedicated to ensuring the registration of current cases rather than to finding retrospective cases (a well-established practice in cancer registration). Hypothetically, if a patient is already dead by the time of reporting to the Diabetes Register, the physician may consider reporting as unnecessary and the patient, consequently, is labeled as a non-diabetic individual. Thus, systematic error is introduced; longer survival is associated with a higher probability that a person with diabetes is labeled as diabetic. Whether this hypothetical scenario explains the atypical findings in our study is difficult to prove or disprove. First, a possible ‘beneficial’ effect of diabetes on cancer patient survival was observed in men but not in women. There is no reason to assume that diabetes registration is more complete for women than men (regarding diagnosed diabetes), as registration depends on the physician, not the patient. Second, even if this scenario is true, this situation applies to a limited number of cases: those who were diagnosed with diabetes before cancer within the same calendar year and who died by the end of that year. To minimize this bias, we restricted patient selection to those reported from 2009 onwards, as the completeness of diabetes registration has improved since 2007 due to regular comparisons with prescription data.
Because we were unable to exclude the hypothetical influence of the delayed registration of diabetes patients, we also examined the association using health service data, although we were aware of several limitations of these data in terms of validity and completeness. First, private health care is not captured by the NHS data. Although private services are not common in cancer or diabetes care, the wealthiest patients and mildest cases could be missed. Second, to minimize the number of potentially incorrect and unconfirmed cancer diagnoses, outpatient services were not included. In Latvia, cancer diagnostics and treatment are mainly concentrated in hospitals; in addition, some hospitals provide specialized oncology care according to specific programs and earmarked funding. Discharge diagnoses from those hospitals were accepted as valid. However, not all cancer patients are referred to specialized hospitals; in practice, elderly people with advanced disease are more likely to be treated in usual non-specialized hospitals or primary care facilities. Thus, our intention to improve the validity of cancer diagnoses inevitably led to patient selection. Indeed, the patients in the NHS data set were on average of two years younger than those in the Cancer Register. If the better short-term survival of diabetic men resulted from the selection of cases from specialized hospitals, the referral to those hospitals should be differential, e.g., a poor prognosis should discourage the hospitalization of diabetic men more than that of non-diabetic men. However, the proportion of diabetic men in the NHS data set was higher compared with that in the disease register data set (8.9 vs. 7.6%), which, in combination with the younger mean age, suggests that diabetes patients were overrepresented in the specialized hospitals. Unfortunately, the absence of cancer stage records in the NHS data prevents comparison of disease severity.
Considering cancer care practices can provide a broader context. Low public financing of health care, which was deepened by the economic crisis of 2008 and consequently, limited access to state-funded health services have been recognized as major threats in Latvia [Citation21,Citation22]. Costs of cancer diagnostics and treatment are covered by the state (although not all new-generation cancer drugs are included), but other barriers exist, such as wait time and distance. In 2016, oncology care was prioritized and a so-called ‘green corridor’ was established to increase the speed of examinations and treatments. Still, informal means (bribes and friendly relations) are also used to advance the process; thus, a person’s knowledge of the health care system’s operation is essential. We speculate that diabetes patients, who already had experience interacting with the health-care system, might have advantages compared with naïve individuals.
Furthermore, diabetes patients must contact their family doctor regularly, which may result in earlier cancer detection. Although one of the proposed mechanisms explaining poorer cancer prognosis in diabetes patients is lower screening uptake [Citation7], it may not be universally applicable. Indeed, some studies have shown higher participation of diabetes patients in colorectal [Citation23] and prostate cancer [Citation24] screenings. Whether diabetes patients are disadvantaged may depend on the prevalence of cancer screening in the general population. In Latvia, population screening programs for breast and cervical cancer were launched in 2009 and that for colorectal cancer was initiated in 2007. In 2015, the participation rates reached 34.9 and 25.0% out of women invited to breast and cervical cancer screenings, respectively and 10.9% of the target population participated in colorectal cancer screening [Citation25]. Although considerable differences in cancer stage distribution were not observed in our study, among men with bladder cancer, prior diabetes showed a beneficial association with short-term survival in both data sets. It seems plausible that regular urine tests, which are common in diabetes patients but rare in non-diabetic men, could reveal hematuria earlier.
If close contact with the health system explains the better short-term prognosis of diabetes patients (surveillance bias), this would also be in line with the gender differences. Latvian population health interview surveys [Citation26,Citation27] have repeatedly shown that men use out-patient health services less than women. In 2008, 59.6 of men and 78.0% of women aged 55–64 had consulted with family doctors within the last 12 months [Citation26] and in a 2014 survey [Citation27], these percentages were 68.2 and 85.6%, respectively. In addition, fewer contacts with health professionals can delay diagnosis of type 2 diabetes in men. According to the Diabetes Register, both the prevalence and number of newly registered type 2 diabetes patients above age 60 are higher in women than in men [Citation13]. If diabetes detection depends on the usage of prophylactic health services, another paradox is possible: people of higher socio-economic status might be more frequently diagnosed with type 2 diabetes than people of lower status, which could consequently modify their future prospects in the case of cancer. The positive association between socioeconomic status and diabetes prevalence has been reported from low- and middle-income countries [Citation28].
Further exploration of potential interaction between socio-economic status, diagnosed diabetes and cancer prognosis is limited due to the absence of socio-economic characteristics of registered patients in Latvia. While data linkage between census data (having socio-economic factors) and disease registers is not on the agenda, the exchange of data on screening examinations and other health services should be facilitated by the long awaited e-health system. It was finally introduced in 2017, although, not completely, but the ultimate goal is to link all existing data bases in one system, allowing more efficient planning and monitoring of health services and health outcomes. If functioning as expected, the e-health system will provide an opportunity to elucidate at least some of mechanisms linking diabetes to cancer outcomes. In addition, more studies from countries undergoing economic transition could support or refute this interpretation and show whether and to what extent our findings are generalizable.
Conclusion
In summary, our study confirmed that preexisting diabetes was associated with worse prognosis among women with cancer, whereas among men, the association was more intriguing, even suggesting a protective effect of diabetes in the short-term. This unusual finding might be explained by the complex interplay between the healthcare system and individuals behaviors. If regular health checkups and contact with health professionals are uncommon among middle-aged men in the general population, those who are diagnosed with diabetes might receive more prophylactic examinations and better management of cancer care. These findings indicate that socioeconomic and organizational contexts are extremely important in interpreting individual studies and even more so for summarizing evidence in systematic reviews and meta-analyses.
Ieva_Strele_et_al._Supplemental_material.pdf
Download PDF (859.7 KB)Acknowledgments
The authors thank the Centre for Disease Prevention and Control and the National Health Service of Latvia for co-operation in providing the research datasets.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Carstensen B, Jørgensen ME, Friis S. The epidemiology of diabetes and cancer. Curr Diab Rep. 2014;14:535.
- Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300:2754–2764.
- Barone BB, Yeh HC, Snyder CF, et al. Postoperative mortality in cancer patients with preexisting diabetes. Systematic review and meta-analysis. Diabetes Care. 2010;33:931–939.
- Stein KB, Snyder CF, Barone BB, et al. Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta-analysis. Dig Dis Sci. 2010;55:1839–1851.
- Cai H, Xu Z, Xu T, et al. Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: a meta-analysis of 11 cohort studies. Diabetes Metab Res Rev. 2015;31:336–343.
- Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29:40–46.
- Renehan AG, Yeh HC, Johnson JA, et al. Diabetes and cancer (2): evaluating the impact of diabetes on mortality in patients with cancer. Diabetologia. 2012;55:1619–1632.
- Mills KT, Bellows CF, Hoffman AE, et al. Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum. 2013;56:1304–1319.
- Zhang D, Zhao Y, Wang T, et al. Diabetes mellitus and long‐term mortality of ovarian cancer patients. A systematic review and meta‐analysis of 12 cohort studies. Diabetes Metab Res Rev. 2017;33:e2868.
- Zanders MM, Vissers PA, Haak HR, et al. Colorectal cancer, diabetes and survival: epidemiological insights. Diabetes Metab. 2014;40:120–127.
- Leon DA. Trends in European life expectancy: a salutary view. Int J Epidemiol. 2011;40:271–277.
- Healthy life years and life expectancy at birth [Internet]. Luxembourg: Eurostat, European Commission; 2017. [cited 2017 July 5]. Available from: http://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&language=en&pcode=tsdph100&plugin =1.
- Public health and morbidity. In: Lepiksone J, editor. Statistical yearbook of health care in Latvia 2015. 17th ed. Riga: Centre for Disease Prevention and Control of Latvia; 2016. p. 67–154.
- Forman D, Bray F, Brewster DH, et al. Cancer Incidence in Five Continents, Vol. X. IARC Scientific Publication No. 164. Lyon: International Agency for Research on Cancer; 2014.
- WHO Cancer Mortality Database [Internet]. Lyon: International Agency for Research on Cancer. 2010 – [cited 2017 Jan 18]. Available from: http://www-dep.iarc.fr/WHOdb/WHOdb.htm.
- DeAngelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5 – a population-based study. Lancet Oncol. 2014;15:23–34.
- Minicozzi P, Innos K, Sanchez MJ, et al. Quality analysis of population-based information on cancer stage at diagnosis across Europe, with presentation of stage-specific cancer survival estimates: a EUROCARE-5 study. Eur J Cancer. 2017;84:335–353.
- Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease. A statement for healthcare professionals from the American heart association. Circulation. 1999;100:1134–1146.
- Ranc K, Jørgensen ME, Friis S, et al. Mortality after cancer among patients with diabetes mellitus: effect of diabetes duration and treatment. Diabetologia. 2014;57:927–934.
- Walker JJ, Brewster DH, Colhoun HM, et al. Cause-specific mortality in Scottish patients with colorectal cancer with and without type 2 diabetes (2000-2007). Diabetologia. 2013;56:1531–1541.
- Karanikolos M, Gordeev VS, Mackenbach JP, et al. Access to care in the Baltic States: did crisis have an impact? Eur J Public Health. 2015;26:236–241.
- Country report Latvia 2016. Commission staff working document. Brussels: European Commission; 2016 [cited 2017 Feb 9]. Available from: http://ec.europa.eu/europe2020/pdf/csr2016/cr2016_latvia_en.pdf.
- Porter NR, Eberth JM, Samson ME, et al. Diabetes status and being up-to-date on colorectal cancer screening, 2012 behavioral risk factor surveillance system. Prev Chronic Dis. 2016;13:e19.
- Dankner R, Boffetta P, Keinan-Boker L, et al. Diabetes, prostate cancer screening and risk of low- and high-grade prostate cancer: an 11 year historical population follow-up study of more than 1 million men. Diabetologia. 2016;59:1683–1691.
- Programmas rezultāti [Results of the program]. Riga: The National Health Service of Latvia [Internet]; 2017. [cited 2017 Feb 13]. Available from: http://www.vmnvd.gov.lv/lv/veselibas-aprupes-pakalpojumi/veza-savlaicigas-atklasanas-programma/programmas-rezultati.
- Svarckopfa A, editor. Health survey results of Latvian population, 2009. Riga: Central Statistics Bureau of Latvia; 2010.
- Svarckopfa A, editor. Iedzīvotāju veselības apsekojuma rezultāti, 2016 [Health Survey Results of Latvian Population, 2016]. Riga: Central Statistical Bureau of Latvia; 2016.
- Hosseinpoor AR, Bergen N, Mendis S, et al. Socioeconomic inequality in the prevalence of noncommunicable diseases in low- and middle-income countries: results from the World Health Survey. BMC Public Health. 2012;12:474.
