Abstract
Background: Low-dose metronomic chemotherapy (LDMC) is an alternative for treatment of patients with late-stage prostate cancer (PC) not susceptible to regular chemotherapy due to its severe side effects. The exact working mechanisms of LDMC have not been established, although anti-angiogenic effects have been identified. In PC, several studies show clinical effects from LDMC but the mode of action and the role of androgen signaling for its effect are not known. In this study, we used a xenograft model to evaluate the effect of LDMC on PC growth in relation to androgen deprivation.
Material and methods: Subcutaneous human castration-resistant PC xenografts were treated with LDMC using cyclophosphamide (CPA). Treatment effect was compared to treatment with maximum tolerated dose (MTD) and also between intact and castrated mice. Microvessel density (MVD), and factors important for angiogenesis were analyzed with immunohistochemistry and real-time-PCR.
Results: Tumors treated with LDMC were 50% smaller than untreated controls. Tumors in non-castrated mice were not affected by LDMC, but in an androgen receptor (AR) negative tumor model, tumor inhibiting effect were seen in both intact and castrated animals, indicating mechanism via AR. MTD resulted in similar growth inhibition as LDMC in castrated mice, but resulted in severe weight loss. Despite that LDMC induced TSP1 mRNA expression, and the hypoxic area in the tumors was slightly increased, no decrease in MVD was detected.
Conclusions: This study shows that a low-dose metronomic scheduling of CPA was as efficient as MTD treatment, and resulted in fewer side effects. It also demonstrates that a functional androgen signaling axis inhibits this effect despite the castration-resistance of the tumor cells. The anti-angiogenic nature of the effect of LDMC could not be confirmed and further studies to elucidate the working mechanism for treatment response are needed.
Introduction
Prostate cancer (PC) is the second most common malignancy world-wide [Citation1]. Treatment options for these patients were limited until 2004 when docetaxel was shown to give an overall survival advantage and an improvement in quality of life [Citation2,Citation3]. In recent years, several new therapeutic options have emerged such as chemotherapy, new hormonal treatments and radionuclide therapy increasing survival, relieving symptoms and increasing quality of life for patients with CRPC [Citation4–6]. However, standard chemotherapy given in a maximum tolerated dose (MTD) causes several major side effects such as neutropenia, fatigue and neuropathy, limiting its use in elderly patients and in patients with severe comorbidity.
The frequent administration of low doses of cytotoxic drugs without interruptions, so-called low-dose metronomic chemotherapy (LDMC) has been shown to decrease tumor growth in mice models through intraperitoneal (i.p.) injection once every third to sixth days. These pioneering studies indicated that the regimen exerted its effect exclusively via killing of endothelial cells, suggesting an antiangiogenic effect [Citation7,Citation8]. However, in recent years, new mechanisms for the LDMC effect have been identified, and today LDMC is considered as a multi-mechanism therapy. Besides possible effects on tumor cells directly, suggested effects include indirect targeting of the tumor vasculature by induction of apoptosis of endothelial cells and increased thrombospondin 1 (TSP1) expression, as well as targeting pro-angiogenic bone-marrow derived cell populations, suppression of hypoxia-inducible factor 1-alfa (HIF1α), and stimulation of the immune system [Citation9]. The involvement of TSP1 was strongly supported by a study where the LDMC treatment effect was abolished in TSP1-null mice [Citation10]. Further support of the mediating role of TSP1 is the TSP1 induction seen after treatment with metronomic cyclophosphamide (CPA) and paclitaxel in a rat tumor model [Citation11].
The advantage of LDMC for patients with cancer is the lower grade of side-effects from the treatment [Citation9]. In PC, the effects of LDMC are poorly investigated. However, convincing PSA declines after LDMC with CPA have been observed, even in heavily pretreated patient groups, including some being resistant to docetaxel, and the grade of side-effect is often low [Citation12–14]. The effect of using LDMC docetaxel (weekly administration) has been verified in terms of biochemical response and both PFS and OS [Citation15]. Also, pain relief and quality of life evaluation definitely demonstrated superiority of the treatment regimen [Citation2,Citation3,Citation15].
Thus, there is increasing evidence for the efficacy of LDMC, but generally there is still a lack of data in terms of phase III trials showing overall survival benefit. Most published trials are phase II trials and there is a need of large studies comparing LDMC to conventional MTD-regimes [Citation16,Citation17].
Patients with CRPC intended for chemotherapy are almost always treated with ADT. It has become clear that even though systemic androgen levels are low in these patients, intratumoral steroid synthesis enable tumor cells to reactivate the androgen receptor (AR) signaling [Citation18]. Despite this, it has been shown that continued ADT confers a survival benefit also in the CRPC state, an effect that may partly be mediated by the androgen-sensitive supporting tumor stroma, both in the prostate and in skeletal metastases. The role of ADT in the possibly stroma-mediated efficacy of LDMC has never been investigated.
In the present study, we aimed at evaluating the effect and possible working mechanisms of metronomically administered CPA in a CRPC xenograft tumor model, as well as the possible influence of castration therapy on its efficacy.
Material and methods
Cell culture
LNCaP-19, a CRPC cell line derived from LNCaP by androgen deprivation, was established in vitro in our laboratory [Citation19]. PC-3 was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in RPMI 1640 with stable l-glutamine (Gibco, Invitrogen Ltd., Paisley, UK) supplemented with 10% fetal bovine serum (FBS, PC-3) or dextrane-charcoal treated (DCC-FBS, LNCaP-19), glucose (2.5 g/L), sodium pyruvate (1 mM), and penicillin–streptomycin.
Animal studies
Male athymic BALB/c-nude mice were obtained from Charles River Laboratories International Inc. (Wilmington, MA, USA). Mice were six to eight weeks when used in the experiment. All animal studies were approved by the Animal Ethics Committee in Gothenburg.
Two million LNCaP-19 cells or one million PC-3 cells were suspended in 200 µl culture medium and matrigel (1:1) (BD Bioscience, Bedford, MA, USA), or in 100 µl culture medium, respectively. The cell suspensions were subcutaneously implanted in the flank of each mouse. To mimic the castration-resistant disease in humans, some groups of mice were castrated via scrotal incision during general anesthesia prior to tumor inoculation.
After approximately two weeks, when the tumors were clearly detectable (approximately 200 mm3), treatment was initiated. For LDMC, CPA (25 mg/kg/day) was administered via drinking water. The LDMC-group was compared to a MTD-group which was treated with CPA (60 mg/kg) i.p. on days 0, 2 and 4 every third week, as well as a control group receiving saline i.p. in the MTD schedule. Doses were based on a pilot study where a MTD dose of 80–100 mg/kg was overly toxic and a metronomic dose of 20 mg/kg showed only limited tumor inhibition (data not shown). The treatment was maintained until termination. Tumors were measured weekly with a caliper and the volume was calculated using the formula length × width2 × 0.5.
The body weight of each mouse was measured twice a week and signs of animal distress were followed continuously, as an evaluation of the side-effects of treatment. Animals were sacrificed if found to be cachexic or if the body-weight loss exceeded 15% after MTD treatment without recovery in the following days. Sixty minutes before sacrifice, the mice were injected with the hypoxia marker pimonidazole (HypoxyProbe, Burlington, MA, USA) (i.p. 60 mg/kg). One part of the tumor was fixed in formalin for paraffin embedding and one part was frozen in liquid nitrogen and stored at −80 °C.
Immunohistochemistry
Tissue sections (4-μm) were deparaffinized and rehydrated. For antigen retrieval, sections were heated in antigen unmasking solution (H-3300; Vector Laboratories, Burlingame, CA, USA) and endogenous peroxidase was quenched in 0.3% hydrogen peroxide in methanol. Immunohistochemistry was performed using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s protocol. Briefly, nonspecific binding was blocked with normal serum and sections were incubated with a specific primary antibody at 4 °C over-night. The following antibodies were used: for MVD, CD34 specific primary antibody, 1:200–1:500, 2 μg/ml, ab8158 (Abcam, Cambridge, UK); for proliferation, antibody against Ki-67, 1:200–1:400, RM-9106-S, (Neomarkers; Thermo Fisher Scientific, Fremont, CA, USA); and antibody against pimomidazole, 1:3000, Hypoxyprobe TM-1 (Burlington, MA, USA). Detection was performed with a biotinylated secondary antibody followed by ABC-HRP reagent. DAB was used for visualization and the sections were counterstained with hematoxylin, dehydrated, and mounted.
Evaluation of immunostaining
Microvessel density (MVD) was evaluated as the number of CD34 positive vessels in the non-necrotic tumor tissue. Vessels were counted at a magnification of 200× in five randomly chosen fields in each section. The analysis were performed independently and blinded by two investigators. The extent of hypoxia was evaluated in the Biopix software (Biopix, Gothenburg, Sweden) as percentage hypoxic area of viable tumor tissue.
RNA preparation and real-time RT-PCR
Tumor tissues were homogenized in TRIzol reagent (Invitrogen Ltd., Paisley, UK) according to the manufacturer’s protocol, and total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). The RNA concentrations were measured using Nanodrop (Thermo Fischer Scientific Inc., Wilmington, DE). A total of 1 μg of total RNA were reversely transcribed into cDNA using the VILO Superscript cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA) in accordance with the manufactures instructions. RT-qPCR was performed with the ABI Prism 7500 Fast Sequence Detector (Applied Biosystems, Foster City, CA, USA), and specific TaqMan probes used were: human TSP1 (Hs00170236_m1), mouse TSP1 (Mm00449032_g1), human VEGFA (Hs00900055_m1), mouse VEGFA (Mm01281449_m1), mouse PECAM1 (Mm01242584_m1), mouse CDH5 (Mm00486938_m1), mouse ENG (Mm00468256_m1) and endogenous controls for human GAPDH (Hs03929097_g1) as well as for mouse GAPDH (Mm99999915_g1), purchased as TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). The PCR parameters were according to the manufacturer’s protocol. For relative mRNA quantification, the ΔΔCt method was used. The expression of target gene was normalized to the endogenous control GAPDH. Values are presented as the means (SEM) and the Mann–Whitney test was used to evaluate differences between the groups, with p ≤ 0.05 considered to indicate statistical significance.
Protein preparation and Western blotting
Total protein was prepared from tumor tissues, by homogenization in a lysis buffer that consisted of PBS (pH 7.5), 0.15 M NaCl, 0.25% Tween 20, protease and phosphatase inhibitors (1x, cOmpleteTM Mini and PhosSTOP, respectively; Roche Diagnostics, Mannheim, Germany). Samples were sonicated, centrifuged and the supernatants were collected. Protein concentrations were measured using the BCA protein assay kit (Pierce, Rockford, IL, USA) according to the manufacturer protocol. 30 μg of reduced protein was loaded on a 4–12% Bis–Tris gradient gel and electrophoresis was performed using MOPS (Invitrogen, Carlsbad, CA, USA) as running buffer and transferred onto polyvinyldifluouride (PVDF) membranes using the iBlot gel transfer system (all above products were purchased from Invitrogen, Carlsbad, CA, USA). Membranes were blocked in 5% dry milk and subsequently incubated with primary antibodies over night at 4 °C.
Primary antibodies used were against CD47, ab175388 (Abcam, Cambridge, UK), CD36, ab137320 (Abcam, Cambridge, UK), TSP1, ab85762 (Abcam, Cambridge, UK) and TSP2, ab112543 (Abcam, Cambridge, UK). As loading control β-actin was used. Membranes were incubated with a horseradish-peroxidase (HRP)-labeled secondary antibody for 1h at room temperature. The immunoreactions were detected using the ECL advanced western blotting detection system (RPN2135; Amersham™; GE Healthcare). Chemiluminescent signals were visualized using a LAS-4000 CCD camera (Fujifilm, Tokyo, Japan).
Results
Metronomic CPA inhibits tumor growth, increases TSP1 expression despite unchanged microvessel density
Low-dose metronomic chemotherapy scheduling has proven to be a less toxic alternative to MTD scheduling for patients with cancer. The efficacy of metronomic chemotherapy was evaluated in castrated mice with subcutaneous LNCaP-19 tumors that were treated with oral CPA.
In this tumor model, there was a significant inhibitory effect on tumor growth of the LDMC treatment as compared to untreated controls (n = 10 in each group). After nine weeks of treatment, the relative tumor volume of the mice given LDMC CPA was 50% of the control tumors (p < .01). The efficacy of CPA administered according to LDMC did not differ from CPA administered according to the MTD schedule (). These results were confirmed in another separate experiment (data not shown).
Figure 1. Tumor growth and animal body weight and relative expression of Thrombospondin-1 (TSP1) mRNA in tumors. (A) Relative volumes of subcutaneous tumors after MTD and LDMC scheduling of CPA compared to untreated controls. Volumes were measured with a caliper and related to their individual volume (approximately 200 mm3) at treatment start (Day 0). (B) Animal body weights after MTD and LDMC scheduling of CPA. Arrows indicate new cycles of MTC treatment on Days 0, 2 and 4 in each treatment cycle, with a new cycle every third week. (C,D) Levels of TSP1 mRNA in tumors treated with MTD and LDMC compared to controls; (C) human TSP1, (D) mouse TSP1. *p < .05; **p < .01.
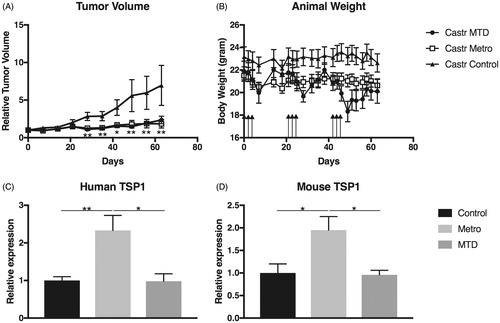
In the metronomic treatment group, none of the animals lost more than 10% of their starting body weight. In contrast, in the MTD group the mice were clearly affected by the treatment. Temporarily, 8 of 10 animals lost more than 10% of their starting body weight due to MTD-treatment, and 6 out of 10 animals lost more than 15% of their starting body weight but did gain fast recovery. After the third course, 5 out of 10 animals were given repeated 0.9% NaCl injections to help improvement. Despite that, after the third course of MTD treatment, 3 of 10 animals had lost more than 15% of their body weight and in lack of fast recovery they were sacrificed due to their poor condition ().
Low-dose metronomic chemotherapy is considered to target the tumor stroma in addition to the tumor cells, and TSP1 has been suggested as a mediator of the anti-angiogenic effects of LDMC in several studies. Therefore, we investigated the expression of TSP1-mRNA in the tumor tissue. In the castrated animals both tumor cell-derived (human) (p < .01 and stroma-cell derived (mouse) TSP1 expression (p = .028) were significantly upregulated in LDMC-treated animals compared to controls as well as compared to MTD treated (p = .011 and p = .011) (). To ensure that TSP1 has the potential to mediate the treatment effect, the presence of the common TSP1-receptors CD36 and CD47 in the tumors was verified by Western blot analyzes. However, the levels of the receptors were not affected by the treatment (data not shown). To assess the effect of LDMC on the neovasculature the microvessel density (MVD) was investigated in the subcutaneous tumors after treatment. In non-necrotic areas of the tumor tissue, in spite of TSP1 upregulation, no statistically significant differences in MVD between the MTD- or LDMC-treated animals could be demonstrated, as compared to the controls ().
Table 1. Microvessel density.
In line with the unaffected MVD, no changes in expression of VE-cadherin (CDH5) or endoglin (ENG) could be detected in any of the experiments (data not shown). Despite the absent effects on MVD in the tumor tissue after nine weeks of treatment, increased hypoxia were indicated in non-necrotic areas of tumors from castrated short-term (2, 5 weeks) LDMC treated animals. The hypoxia increased from 5% in the controls (n = 6) to 10% in LDMC treated mice (n = 10) after this short treatment, but this result did not reach statistical significance (p = .129; data not shown). In the same set-up, no changes in necrotic areas could be detected.
The tumor inhibiting effect of LDMC is inhibited by a functional hypothalamus–pituitary–gonadal axis
Since the efficacy of androgen-targeting drugs, such as abiraterone and enzalutamide, demonstrates the persistent importance of androgens also in CRPC, the influence of castration on LDMC efficacy was evaluated in mice with subcutaneous LNCaP-19 tumors.
In the castrated animals, the previously shown inhibitory effect on tumor growth was verified. The body weights of the animals were stable and similar for all groups (data not shown). At the end of experiment the tumor volume in the LDMC-treatment group (n = 9) was 60% of that of the controls (n = 7) (p = .013) (). Interestingly, no inhibitory effect on tumor growth was seen in the non-castrated animals (LDMC n = 9; Controls n = 8) (), indicating that the addition of castration to the LDMC treatment is critical for the inhibiting effect on tumor growth due to LDMC treatment in this CRPC tumor model. However, in the tumor tissue from non-castrated animals there was an upregulation of the tumor cell-derived TSP1 (p = .011) but not stroma cell-derived TSP1 (p = .20), despite that no inhibition of tumor growth was seen in this group. A small upregulation of mouse and human TSP1 was also seen in tumor tissue from castrated animals but it was not statistically significant (p = .142 and p = .153) ().
Figure 2. Effect of castration on LDMC tumor inhibition and TSP1 expression. Tumor volumes were measured with a caliper and related to their individual volume (approximately 200 mm3 for LNCaP-19 and 70 mm3 for PC-3) at treatment start (Day 0). (A, B) Relative volumes of LDMC treated LNCaP-19 tumors compared to controls in (A) castrated and (B) intact animals. (C) Human and (D) mouse TSP1 levels in LDMC-treated LNCaP-19 tumors compared to controls in castrated and intact animals. (E,F) Relative volumes of LDMC treated PC-3 tumors compared to controls in (E) castrated and (F) intact animals. *p < .05; **p < .01.
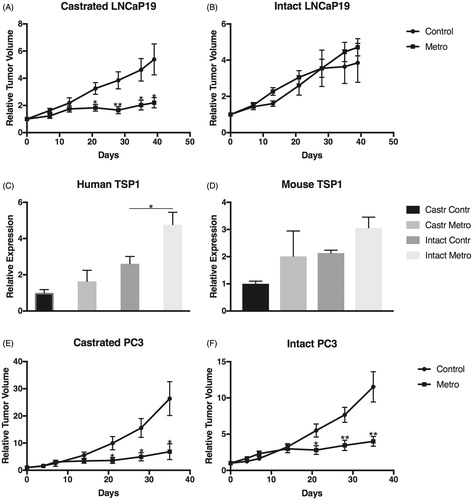
Interestingly, and in line with the TSP1 results, LDMC resulted in a significantly decreased MVD in non-castrated animals, albeit the absence of tumor growth inhibition in this group. As in the previous experiment, no difference in MVD was seen in the castrated animals ().
To assess whether the difference in response between castrated and intact mice was mediated by AR signaling in the stroma or the tumor cells, another CRPC cell line, PC-3, without expression of AR was used. In that experiment, LDMC significantly inhibited tumor growth regardless of castration () (Castrated n = 4; Controls n = 9), indicating that the lack of growth inhibition of LNCaP-19 in intact mice is due to a stimulatory effect on the tumor cells via its AR, and not mediated by an androgen-responsive stroma.
Discussion
Metronomic treatment with cytotoxic drugs has been suggested as a less toxic strategy for patients with cancer, although the efficacy of this regimen for patients with castration-resistant PC is not clear. In the present study, we show that LDMC CPA has a robust inhibiting effect on the growth of subcutaneously implanted castration-resistant prostate tumors in castrated mice with less severe side-effects.
In our castration-resistant model, LDMC resulted in a decreased tumor growth in castrated mice, reflecting the clinical situation where patients continue ADT even though a castration-resistant disease has relapsed. The tumor inhibitory effect was comparable to that obtained with traditional MTD scheduling. Our study also demonstrates that in spite of the fact that the LDMC-treated animals received a higher dose of the drug over time than did the MTD-treated animals, LDMC results in a lower grade of side-effects, as evidenced by the stabilization of mouse body weight over time, as compared to the severe weight loss observed in the MTD-treated animals. In line with our results, Man et al. previously showed that CPA MTD-treatment in tumor bearing mice caused a sudden weight loss and death, whereas the CPA LDMC treatment showed tolerability and stable weight curves [Citation20]. Analogous, such pattern has been observed in the context of LDMC treatment in the clinical setting, where LDMC-treated patients also receive a higher total dose of drug over time than MTD-treated patients, and still tolerate the treatment better. It is highlighted in the literature that consensus about the LDMC dosing is missing and that there is a pressing need for pharmacokinetic studies [Citation21].
Despite the increasing use of chemotherapy for metastatic PC, both in the CRPC disease and, more recently, in the castration-sensitive setting [Citation22,Citation23], the impact of androgen deprivation on chemotherapy efficacy is not thoroughly investigated. The present study demonstrates that LDMC using CPA inhibited the growth of LNCaP-19 CRPC tumors only in combination with castration. However, this castration-dependent difference in efficacy was not observed using another CRPC cell line without AR expression. Thus, it can be concluded that any AR mediated effects in the tumor stroma is not the reason for the castration-dependent growth inhibition of LNCaP-19. One explanation for the absent tumor inhibition in the non-castrated setting, is that stimulation of the still AR-positive CRPC tumor cells by testosterone overrides the inhibitory effect from the microenvironment induced by LDMC. However, castration itself, without LDMC, did not affect LNCaP-19 tumor growth.
The early studies on metronomic treatment convincingly argues in favor of the anti-angiogenic mechanism of action [Citation7,Citation8], but later studies have emphasized that also other mechanisms could be important [Citation9]. In line with the anti-angiogenic theory, our study demonstrates an increase of TSP1 expression as a possible mediator of an anti-angiogenic response to treatment, but in contrast to the anticipated effect on TSP1, we could not detect any significant decrease of the tumor microvasculature. However, the immunohistochemical analysis of blood vessels was performed in the still viable rim of tumor tissue and not in the large necrotic areas of the tumors representing the targeted and dead tumor cells, why care must be taken in interpretation of these data. Despite the lack of robust effects on MVD, tendencies toward increased tissue hypoxia were seen in the treated tumors, especially after short treatment. Taken together, the induction of TSP1 and the tendency to increased hypoxia in our studies support the notion that anti-angiogenic mechanisms are involved in LDMC effects. However, the lack of tumor inhibition seen in the non-castrated study group despite both TSP1 induction and decreased microvasculature, indicate that the anti-angiogenic effects are not sufficient for efficient tumor growth inhibition by LDMC. Additional modes of action could involve the immune system, since it has been shown that LDMC CPA induces increased numbers of dendritic cells, NK cells and macrophages [Citation24,Citation25].
In conclusion, the present study shows that LDMC has evident tumor inhibitory effects in CRPC tumors, with a favorable toxicity profile. This is important, since some of the patients in this state of the disease have poor physical status and may not tolerate the toxicity of traditional MTD scheduling. In addition, the results show that castration is necessary for the tumor growth inhibition of LDMC with CPA. However, the working mechanisms of LDMC in PC needs to be further investigated, since this study did not convincingly confirm its anti-angiogenic effect.
Acknowledgments
The authors would like to thank Karin Larsson and Anita Fae for technical assistance during laboratory work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512.
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520.
- Afshar M, Evison F, James ND, et al. Shifting paradigms in the estimation of survival for castration-resistant prostate cancer: a tertiary academic center experience. Urol Oncol. 2015;33:338.e1–337.
- Vale CL, Burdett S, Rydzewska LH, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17:243–256.
- Fitzpatrick JM, Bellmunt J, Fizazi K, et al. Optimal management of metastatic castration-resistant prostate cancer: highlights from a European Expert Consensus Panel. Eur J Cancer. 2014;50:1617–1627.
- Klement G, Baruchel S, Rak J, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24.
- Browder T, Butterfield CE, Kraling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886.
- Andre N, Carre M, Pasquier E. Metronomics: towards personalized chemotherapy? Nat Rev Clin Oncol. 2014;11:413–431.
- Bocci G, Francia G, Man S, et al. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA. 2003;100:12917–12922.
- Damber JE, Vallbo C, Albertsson P, et al. The anti-tumour effect of low-dose continuous chemotherapy may partly be mediated by thrombospondin. Cancer Chemother Pharmacol. 2006;58:354–360.
- Nelius T, Rinard K, Filleur S. Oral/metronomic cyclophosphamide-based chemotherapy as option for patients with castration-refractory prostate cancer: review of the literature. Cancer Treat Rev. 2011;37:444–455.
- Derosa L, Galli L, Orlandi P, et al. Docetaxel plus oral metronomic cyclophosphamide: a phase II study with pharmacodynamic and pharmacogenetic analyses in castration-resistant prostate cancer patients. Cancer. 2014;120:3923–3931.
- Porsch M, Ulrich M, Wendler JJ, et al. A randomised phase II trial comparing docetaxel plus prednisone with docetaxel plus prednisone plus low-dose cyclophosphamide in castration-resistant prostate cancer. Chemotherapy. 2014;60:129–134.
- Fossa SD, Jacobsen AB, Ginman C, et al. Weekly docetaxel and prednisolone versus prednisolone alone in androgen-independent prostate cancer: a randomized phase II study. Eur Urol. 2007;52:1691–1698.
- Munzone E, Colleoni M. Clinical overview of metronomic chemotherapy in breast cancer. Nat Rev Clin Oncol. 2015;12:631–644.
- Nelius T, Klatte T, de Riese W, et al. Clinical outcome of patients with docetaxel-resistant hormone-refractory prostate cancer treated with second-line cyclophosphamide-based metronomic chemotherapy. Med Oncol. 2010;27:363–367.
- Katsogiannou M, Ziouziou H, Karaki S, et al. The hallmarks of castration-resistant prostate cancers. Cancer Treat Rev. 2015;41:588–597.
- Gustavsson H, Welen K, Damber JE. Transition of an androgen-dependent human prostate cancer cell line into an androgen-independent subline is associated with increased angiogenesis. Prostate. 2005;62:364–373.
- Man S, Bocci G, Francia G, et al. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–2735.
- Bouche G, Andre N, Banavali S, et al. Lessons from the Fourth Metronomic and Anti-angiogenic Therapy Meeting, 24–25 June 2014, Milan. Ecancermedicalscience. 2014;8:463.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177.
- Doloff JC, Waxman DJ. VEGF receptor inhibitors block the ability of metronomically dosed cyclophosphamide to activate innate immunity-induced tumor regression. Cancer Res. 2012;72:1103–1115.
- Chen CS, Doloff JC, Waxman DJ. Intermittent metronomic drug schedule is essential for activating antitumor innate immunity and tumor xenograft regression. Neoplasia. 2014;16:84–96.
