Introduction
Nasopharyngeal adenoid cystic cancer (NACC) is a rare histologic subtype of nasopharyngeal carcinoma (NPC), representing less than 1% of all nasopharyngeal cancers [Citation1,Citation2]. As such, there are currently very little data regarding treatment approaches such as radiotherapy (RT) alone, surgery alone or combined modality therapy (CMT, surgery + RT).
There are 11 clinical studies of NACC, totaling 152 cases, reported to date [Citation3–13]. The two largest single-institutional studies demonstrated conflicting conclusions. One series of 26 patients [Citation7] demonstrated a significant overall survival (OS) benefit for patients receiving CMT versus RT alone. However, another study of 36 patients [Citation9] demonstrated no differences in OS between CMT and RT alone. As a result, there remains a lack of clear consensus.
This study had multiple goals as the first large-volume investigation into the treatment patterns of NACC in the United States. We first addressed clinical practice patterns to ascertain factors associated with the delivery of each paradigm (RT alone, surgery alone or CMT). Temporal trends in use of each modality were also noted, as was OS with each management approach. Although challenging to assess with even multi-institutional analyses owing to the rarity of NACC, the National Cancer Data Base (NCDB) provides a unique resource with which to address these novel but clinically important issues.
Material and methods
The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, which consists of de-identified information regarding tumor characteristics, patient demographics and patient survival for approximately 70% of cases in the United States population [Citation14–38]. The NCDB was designed for patterns of care studies at the local hospital level, containing information not included in the Surveillance, Epidemiology and End Results database, including details regarding surgical approach, systemic adjuvant therapy information, socioeconomic status, driving distance, hospital identifiers and hospital characteristics [Citation39,Citation40]. The data used in the study were derived from a de-identified NCDB file (2004–2013). The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or a statistical methodology employed nor the conclusions drawn from these data by the investigators. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
Inclusion criteria for this study were patients with newly diagnosed, histologically confirmed NACC (NCDB code 8200) treated by RT alone, or surgery with or without RT. Patients receiving any form of surgery were included, such as local tumor-directed surgery (Surgery of Primary Site Codes 14, 20, 22, 25, 26, 27, 28), pharyngectomy (codes 30, 31, 32, 40, 42, 43, 50, 51) or unspecified surgery (code 90). This ‘lumping’ was intentionally performed similar to prior aforementioned retrospective reports owing to the rarity of this disease [Citation3–13]. Exclusion criteria were palliative care treatment (as designated by the NCDB), along with no/unknown RT/surgical treatment. In accordance with the variables in NCDB files, information collected on each patient broadly included demographic, clinical and treatment data.
All statistical tests were performed with SAS software (Version 9.4, Cary, NC, USA); tests were two-sided, with a threshold of p < .05 for statistical significance. For categorical data, chi-squared or Fisher’s exact tests were used to assess differences in patient, tumor and treatment characteristics between each of the three treatment groups. For continuous data, one-way analysis of variance or non-parametric Kruskal-Wallis tests were used for comparison when appropriate. Survival analysis (performed using a Kaplan-Meier methodology) evaluated OS, defined as the interval between the date of diagnosis and the date of death or censored at last contact. Univariate and multivariate Cox proportional hazards modeling evaluated factors predictive of OS. The proportional hazards assumptions in the Cox models were met. The backward elimination method was used to select the most important covariates for the multivariate model. The least significant variable was removed at each step until all remaining variables were significant at p < .05 level.
Results
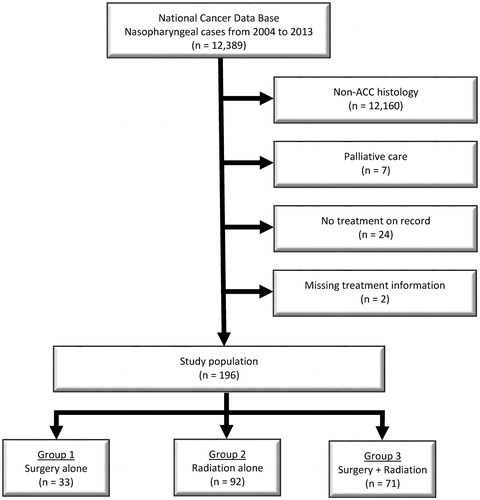
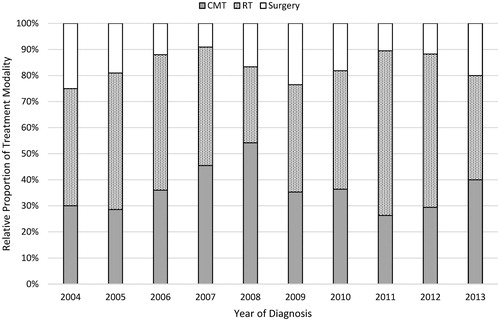
A flow diagram of patient selection is provided in . In total, 196 patients met study criteria, with 92 (47%) receiving RT alone, 33 (17%) receiving surgery alone, and 71 (36%) receiving CMT. demonstrates the normalized temporal trends in treatment paradigm, with no clearly discernible unidirectional trend.
Figure 2. Temporal trends in treatment modality. CMT: Combined modality therapy; RT: radiation therapy.
displays notable patient characteristics. Patients who underwent surgery alone were less likely to receive chemotherapy (p < .001) and receive treatment at an academic facility (p = .028). Patients who were uninsured more often received RT alone, with private insurance more likely associated with receipt of surgery (p = .012). Although there was a statistically significant difference in tumor grade between cohorts (p = .008), the large proportion of unknown values limits any meaningful interpretation.
Table 1. Characteristics of the overall cohort and factors associated with each treatment paradigm.
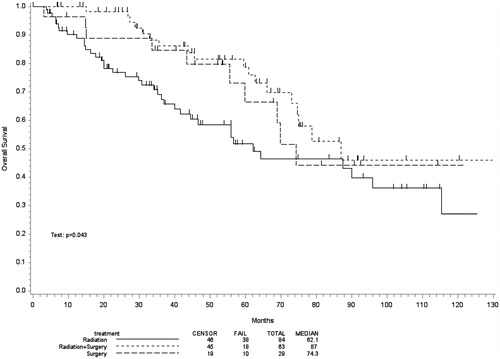
Kaplan-Meier estimates comparing OS in patients undergoing RT alone, surgery alone or CMT are illustrated in . The median survival for the respective groups was 62 (95% confidence interval (CI) 42–115), 74 (95% CI 56-not reached) and 87 months (95% CI 73-not reached), respectively (p = .037).
After univariate analysis was performed in , multivariate analysis revealed that independent predictors of survival were related to race and treatment location (). Additionally, the presence of nodal involvement independently predicted for shorter OS (hazard ratio (HR) 5.45, 95% CI 1.68–17.64, p = .005), as did the presence of distant metastasis (HR 8.35, 95% CI 1.83–38.02, p = .006). Of note, as compared to treatment with RT alone, surgery (HR 0.21, 95% CI 0.06–0.75, p = .016) and CMT (HR 0.25, 95% CI 0.09–0.66, p = .005) were associated with longer OS.
Table 2. Univariate Cox proportional hazards model for OS.
Table 3. Multivariate Cox proportional hazards model for OS.
Discussion
To our knowledge, this is the first report assessing treatment options for NACC; the sample size in this report is larger than that of all published literature to date put together. Our study of a large, contemporary national database of this rare disease most notably demonstrates that treatment with surgical-based options are associated with longer OS. We also show that receipt of a particular treatment paradigm is influenced by factors such as the treating facility and insurance status.
There are several important reflections from these findings. First, treatment with RT alone was significant for a shorter median survival (p = .037). One major caveat to interpreting these data is that the NCDB does not contain information which was used to help guide decision-making for a given treatment modality. Therefore, it is reasonable that RT alone may have been given in a higher risk population with more advanced, unresectable disease or with comorbidities which precluded patients from surgery but that might have influenced OS. Notably, though, there were no significant differences in Charlson-Deyo score or age between groups, although other factors such as performance status are not captured by the NCDB and cannot be accounted for. There was also an increased utilization of chemotherapy (p < .001) as well as a trend toward more cT4 tumors in the RT alone group (p = .088), suggesting potentially higher risk disease. Additionally, patients receiving RT alone were more numerically likely to be uninsured (p = .012) and of black race (trend; p = .080). This raises concern especially because the latter was independently associated with OS as well.
Second, patients who underwent surgery alone were less likely to receive chemotherapy. It is plausible that the lower propensity for nodal dissemination (as compared to other histologies of NPC) contributes to the lack of clear demonstrable benefit for chemotherapy in NACC. In fact, our study demonstrated a 10% degree of nodal involvement, which is consistent with historical reports that range anywhere from approximately 4% to 15% [Citation5,Citation7–9], and clearly lower than NPC (64–88%) [Citation41–43]. This study failed to demonstrate higher OS with chemotherapy, which is to be expected as chemotherapy is presumably part of palliative treatment in most cases [Citation44].
Although the NCDB provides a unique platform with which to study this important clinical question, this investigation is not without limitations. First, despite this being by far the largest such study to date, a limited overall sample size was analyzed simply due to the rarity of NACC. This naturally leads to the lumping of disparate patients with differing characteristics (including nodal positivity, metastatic disease, etc.) into the same analysis, which is not different from that performed in other retrospective reports. This can be a major cause of the discrepancies between published series, including this one. Although our conclusions are in keeping with some prior reports [Citation7], it is readily acknowledged that there is little consensus in the literature mainly owing to retrospective selection biases that are always present. Second, although the NCDB provides information on tumor size, lymphovascular invasion, margin status and grade, this information was omitted or unknown in a large majority of patients and, therefore, was not amenable to rigorous statistical analysis. One additional factor of interest in NACC is perineural invasion (PNI). It is known that NACC is associated with PNI, with cranial nerve invasion reported from 27% to 58% of patients [Citation5,Citation7,Citation9]. However, PNI is also not tabulated in the NCDB. Therefore, it was not possible to determine patterns of care in relation to these clinically important factors. Furthermore, the NCDB also does not provide details such as RT field design/volumes/techniques, or qualitative extent of resection (e.g., based on post-treatment evaluation). It is also plausible that the NCDB does not capture a true population distribution, as the NCDB is not a population database.
Disclosure statement
This study has not been presented or published in part or full form elsewhere. All authors declare no conflicts of interest.
Funding
There was no research support for this study.
References
- Chang ET, Adami H. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777.
- He JH, Zong YS, Luo RZ, et al. Clinicopathological characteristics of primary nasopharyngeal adenocarcinoma. Ai Zheng. 2003;22:753–757.
- Yin ZY, Wu XL, Hu YH, et al. Cylindroma of the nasopharynx: a chronic disease. Int J Radiat Oncol Biol Phys. 1986;12:25–30.
- Lee DJ, Smith RR, Spaziani JT, et al. Adenoid cystic carcinoma of the nasopharynx. Case reports and literature review. Ann Otol Rhinol Laryngol. 1985;94:269–272.
- Wang C, See L, Hong J, et al. Nasopharyngeal adenoid cystic carcinoma: five new cases and a literature review. J Otolaryngol. 1996;25:399–403.
- Schramm VL, Imola MJ. Management of nasopharyngeal salivary gland malignancy. Laryngoscope. 2001;111:1533–1544.
- Liu T. Adenoid cystic carcinoma of the nasopharynx: 27-year experience. Laryngoscope. 2008;11:1988–1988.
- Wen SX, Tang PZ, Xu ZG, et al. Therapeutic modalities of nasopharyngeal adenoid cystic carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2006;41:359–361.
- Cao C, Luo J, Xu G, et al. Management of nasopharyngeal adenoid cystic carcinoma. J Oral Maxillofac Surg. 2013;71:e203–e209.
- Gentile MS, Yip D, Liebsch NJ, et al. Definitive proton beam therapy for adenoid cystic carcinoma of the nasopharynx involving the base of skull. Oral Oncol. 2017;65:38–44.
- Takagi M, Demizu Y, Hashimoto N, et al. Treatment outcomes of particle radiotherapy using protons or carbon ions as a single-modality therapy for adenoid cystic carcinoma of the head and neck. Radiother Oncol. 2014;113:364–370.
- Samant S, van den Brekel Michiel W, Kies MS, et al. Concurrent chemoradiation for adenoid cystic carcinoma of the head and neck. Head Neck. 2012;34:1263–1268.
- Ko JJ, Siever JE, Hao D, et al. Adenoid cystic carcinoma of head and neck: clinical predictors of outcome from a Canadian centre. Curr Oncol. 2016;23:26.
- Bilimoria KY, Stewart AK, Winchester DP, et al. The national cancer data base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690.
- Moreno AC, Verma V, Hofstetter WL, et al. Patterns of care and treatment outcomes of elderly patients with stage I esophageal cancer: analysis of the national cancer data base. J Thorac Oncol. 2017;12:1152–1160.
- Stahl JM, Corso CD, Verma V, et al. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer. 2017;103:11–16.
- Verma V, McMillan MT, Grover S, et al. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97:146–154.
- Bilimoria KY, Bentrem DJ, Stewart AK, et al. Comparison of commission on cancer–approved and–nonapproved hospitals in the united states: implications for studies that use the national cancer data base. J Clin Oncol. 2009;27:4177–4181.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the national cancer database for outcomes research: a review. JAMA Oncol. 2017;3:1722–1728.
- Bott MJ, Patel AP, Verma V, et al. Patterns of care in hilar node-positive (N1) non-small cell lung cancer: a missed treatment opportunity? J Thorac Cardiovasc Surg. 2016;151:1549–1558.
- Haque W, Verma V, Butler EB, et al. Patterns of care and outcomes of multi-agent versus single-agent chemotherapy as part of multimodal management of low grade glioma. J Neurooncol. 2017;133:369–375.
- Haque W, Verma V, Butler EB, et al. National practice patterns and outcomes for T4b urothelial cancer of the bladder. Clin Genitourin Cancer. 2017 [cited 2017 Sep 6]. DOI:10.1016/j.clgc.2017.08.013
- McMillan MT, Ojerholm E, Verma V, et al. Radiation treatment time and overall survival in locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;98:1142–1152.
- Verma V, Ryckman JM, Simoe CB 2nd, et al. Patterns of care and outcomes with the addition of chemotherapy to radiation therapy for stage I nasopharyngeal cancer. Acta Oncol. 2017 [cited 2017 Jul 19]; [5 p.]. DOI:10.1080/0284186X.2017.1351039
- Verma V, Ahern CA, Berlind CG, et al. National Cancer Database Report on pneumonectomy versus lung-sparing surgery for malignant pleural mesothelioma. J Thorac Oncol. 2017;12:1704–1714.
- Haque W, Vema V, Fakhreddine M, et al. Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: analysis of the National Cancer Data Base. Gynecol Oncol. 2017;144:28–33.
- Haque W, Verma V, Butler EB, et al. Definitive chemoradiation at high volume facilities is associated with improved survival in glioblastoma. J Neurooncol. 2017;135:173–181.
- Haque W, Verma V, Butler EB, et al. Radical cystectomy versus chemoradiation for muscle-invasive bladder cancer: impact of treatment facility and sociodemographics. Anticancer Res. 2017;37:5603–5608.
- Haque W, Verma V, Butler EB, et al. Radiation dose in neoadjuvant chemoradiation therapy for esophageal cancer: patterns of care and outcomes from the National Cancer Data Base. J Gastrointest Oncol. 2017. DOI:10.21037/jgo.2017.09.12
- Haque W, Verma V, Butler EB, et al. Addition of chemotherapy to hypofractionated radiotherapy for glioblastoma: practice patterns, outcomes, and predictors of survival. J Neurooncol. 2017 [cited 2017 Oct 31]. DOI:10.1007/s11060-017-2654-y
- Verma V, Allen PK, Simone CB 2nd, et al. Association of treatment at high-volume facilities with survival in Patients Receiving Chemoradiotherapy for Nasopharyngeal Cancer. JAMA Otolaryngol Head Neck Surg. 2017 [cited 2017 Nov 2]. DOI:10.1001/jamaoto.2017.1874
- Verma V, Allen PK, Simone CB 2nd, et al. Addition of definitive radiotherapy to chemotherapy in patients with newly diagnosed metastatic nasopharyngeal cancer. J Natl Compr Canc Netw. 2017;15:1383–1391.
- Haque W, Verma V, Butler EB, et al. Chemotherapy versus chemoradiation for node-positive bladder cancer: practice patterns and outcomes from the National Cancer Data Base. Bladder Cancer. 2017;3:283–291.
- Haque W, Verma V, Bernicker E, et al. Management of pathologic node-positive disease following initial surgery for clinical T1-2 N0 esophageal cancer: patterns of care and outcomes from the national cancer data base. Acta Oncol. 2017 [cited 2017 Nov 30]; [8 p.]. DOI:10.1080/0284186X.2017.1409435
- Haque W, Lewis GD, Verma V, et al. The role of adjuvant chemotherapy in locally advanced bladder cancer. Acta Oncol. 2017 [cited 2017 Dec 11]; [7 p.]. DOI:10.1080/0284186X.2017.1415461
- Verma V, Sukar SM, Brooks ED, et al. Chemoradiotherapy versus chemotherapy alone for unresected nonmetastatic gallbladder cancer: National Practice Patterns and Outcomes. J Natl Compr Canc Netw. 2018;16:59–65.
- Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018. DOI:10.1002/hed.24978
- Haque W, Verma V, Naik N, et al. Metaplastic breast cancer: practice patterns, outcomes, and the role of radiotherapy. Ann Surg Oncol. 2018. DOI:10.1245/s10434-017-6316-2
- Mohanty S, Bilimoria KY. Comparing national cancer registries: the national cancer data base (NCDB) and the surveillance, epidemiology, and end results (SEER) program. J Surg Oncol. 2014;109:629–630.
- Lerro CC, Robbins AS, Phillips JL, et al. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20:1759–1765.
- King AD, Ahuja AT, Leung S, et al. Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck. 2000;22:275–281.
- Ng S, Chang JT, Chan S, et al. Nodal metastases of nasopharyngeal carcinoma: patterns of disease on MRI and FDG PET. Eur J Nucl Med Mol Imaging. 2004;31:1073–1080.
- Sun Y, Ma J, Lu TX, et al. Regulation for distribution of metastatic cervical lymph nodes of 512 cases of nasopharyngeal carcinoma. Ai Zheng. 2004;23:1523–1527.
- Laurie SA, Ho AL, Fury MG, et al. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. 2011;12:815–824.