Abstract
Background: The optimal primary external beam radiation therapy (EBRT) radiation schedule for malignant epidural spinal cord compression (MSCC) remains to be determined. The ICORG 05-03 trial assessed if a 10 Gy single fraction radiation schedule was not inferior to one with 20 Gray (Gy) in five daily fractions, in terms of functional motor outcome, for the treatment of MSCC in patients not proceeding with surgical decompression. This article reports on two of the secondary endpoints, Quality of life (QoL), assessed according to the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30) version 3.0 (EORTC Data Center, Brussels, Belgium) and pain control assessed using a visual analog scale.
Methods: A randomized, parallel group, multicenter phase III trial was conducted by Cancer Trials Ireland (formerly All-Ireland Cooperative Oncology Research Group, ICORG), across five hospital sites in Ireland and Northern Ireland. Patients were randomized to 10 Gy single fraction of EBRT or 20 Gy in five fractions in a 1:1 ratio. Patients with baseline and 5-week follow up QoL data are included in this analysis.
Findings: From 2006 to 2014, 112 eligible patients were enrolled for whom 57 were evaluated for this secondary analysis. After adjusting for pre-intervention scores, there was no statistically significant difference in post-treatment Summary scores (excl. FI and QL), or pain scores between the two RT schedules at 5 weeks and 3 months following EBRT. There was a statistically significant relationship between the pretreatment and post-treatment Summary scores (p = .002) but not between the pre-treatment and post-treatment pain scores.
Interpretation: Primary radiotherapy for the treatment of MSCC significantly improves QoL in patients not proceeding with surgical decompression. After adjusting for pre-intervention scores, there was no statistically significant difference between a 10 Gy single fraction radiation schedule and one with 20 Gy in five daily fractions on post-treatment QoL Summary scores. For most patients, an effective treatment with low burden would be desirable. A single fraction schedule should be considered for this group of patients.
Introduction
Malignant spinal cord compression (MSCC) is one of the most devastating complications of cancer, usually presenting with a history of progressive pain, paralysis, sensory loss and loss of sphincter control [Citation1,Citation2]. This leads to a substantial and inevitably striking change in quality of life (QoL) for patients and their families. From its first description by Spiller [Citation1], MSCC continues to be an emergency commonly requiring rapid decision making on the part of several specialists. Back pain is the earliest and most common symptom, presenting in more than 95% at diagnosis [Citation3,Citation4]. The median time from pain onset to MSCC diagnosis is about 2 months [Citation3,Citation5].
Standard management involves high dose steroids in combination with either decompressive surgery followed by radiotherapy (RT) or RT alone. Despite a randomized trial by Patchell et al. showing a superior functional outcome when using a surgical-based strategy in combination with RT versus RT alone [Citation6], most patients are presently treated by primary RT as it is difficult to justify the risks of surgical treatment for patients without a significant life expectancy. However, there is still debate regarding the most appropriate RT schedule. Various radiation schedules have been used, ranging from mildly hypofractionated schedules (10–15 daily fractions and 5 weekly fractions) to single fraction (SF) radiotherapy. A commonly used schedule is 20 Gray (Gy) in five fractions. Despite multiple studies showing equal survival outcomes with SF radiotherapy [Citation7,Citation8], surveys of the patterns of practice involving radiation oncologists worldwide consistently show a reluctance to adopt SF as a standard or preferred practice [Citation9]. It is important to note that different trials can use different definitions of MSCC and as such, differing results can be difficult to interpret.
MSCC occurs in the setting of widespread cancer and large retrospective studies have found the median survival time to be 3–6 months [Citation10,Citation11]. As such, reduction of the treatment burden faced by these patients should be paramount and a certain focus should be placed on QoL. The transport to the RT department and the positioning on the treatment couch may cause discomfort for the paraparetic patient [Citation12,Citation13] and multiple fractions of radiotherapy (RT) obviously necessitate either a longer inpatient stay or repeated trips to the treatment facility. For this group of patients, the majority of whom have a very short survival, prolonged fractionation schedules will take up a substantial portion of the remainder of their life. Thus, we must consider the merits of SF radiotherapy.
Purpose/objective
The primary endpoint of this Irish Cooperative Oncology Research Group (ICORG) 05-03 trial was to determine if a 10 Gy single fraction is not inferior to the standard 20 Gy in five fractions, in terms of functional motor outcome for the treatment of MSCC. Secondary objectives were to analyze QoL, acute/late radiation-induced toxicity, pain control, sphincter function and overall survival.
The primary outcome data was presented at the American Society for Radiation Oncology (ASTRO) 2014 meeting and showed non-inferiority [Citation14]. An article dealing with the primary endpoint is under consideration for publication at present. This article reports on two of the secondary endpoints, QoL, assessed according to the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30) version 3.0 (EORTC Data Center, Brussels, Belgium) [Citation15,Citation16] and pain control, assessed using a visual analog scale (VAS). It compares outcomes in patients with MSCC not proceeding with surgical decompression and treated by primary RT with one of two external beam radiation therapy (EBRT) fractionation schedules (FS).
This article analyses those eligible patients with available QoL data at baseline and at 5 weeks and sought to answer two main questions:
Is RT improving QoL?
Is there a difference in QoL outcomes between fractionation schedules?
We also performed a subgroup analysis to examine the baseline characteristics of patients who died prior to their 5-week follow-up in order to identify those patients who possibly should not receive RT due to their extremely short life expectancy. We briefly examined patient compliance with the EORTC QLQ C30 questionnaire, and thus its usefulness in a palliative patient population.
Methods
Study design
This was a parallel-group study conducted at five sites in Ireland. The Trial ID is ICORG 05-03 (also NCT00968643 and EU-20952) Patients were randomly assigned to one of two parallel groups in 1:1 ratio, to receive either 10 Gy of EBRT in a single fraction, or a control fractionation regimen of 20 Gy in five daily fractions. ICORG 05-03 was an ICH-GCP compliant prospective non-inferiority phase III trial. The study was carried out in compliance with the ICORG protocol, Good Clinical Practice Guidelines and applicable ethical standards. An independent Data Monitoring Committee reviewed unblinded data for patient safety; no interim analyses for efficacy or futility were done. Its review of safety figures between experimental and control patients found no safety concerns with the trial intervention.
The study measured the change in the patients’ mobility at five weeks based on a modified Tomita scoring system. The modified Tomita score evaluated patient mobility using a three-class scale, with class I indicating the patient was mobile and unaided; class II indicating the patient was mobile with a walking aid; and Class III indicating the patient was bed-bound (which corresponds to the original Tomita scale class III and IV). It was judged that a detrimental change of 0.4 units in the mobility scale between the baseline and five-week assessments would be unacceptable. For this non-inferiority study to have a power of 80% for a test using a one-sided 95% confidence limit, that the difference in the mobility change between the arms will not include −0.4, required 76 evaluable patients overall. Evaluable patients were defined as those alive and with a documented mobility status at 5 weeks. A sample size of approximately 126 patients was necessary, given an anticipated loss to follow-up of 40%. The Independent Data Monitoring Committee recommended that the study cease recruitment as soon as the necessary number of evaluable patients had been reached.
Participants
Eligible participants were all adults aged 18 or over with MRI-documented MSCC/cauda equina (MRI of the entire spine performed), histologically proven malignancy (other than leukemia, myeloma, lymphoma, germ cell tumors, or primary tumors of the spine or vertebral column), Karnofsky performance status (KPS) ≥ 30 and written informed consent. In order to fulfill the definition of MSCC, patients were required to be symptomatic with radiological presence of a mass that touches, displaces, indents the spinal cord, or leads to complete loss of definition of spinal cord. Patients with two compression levels were eligible for inclusion. Exclusion criteria were previous irradiation of the relevant spinal segment, solitary bone metastasis with controlled primary site or patients deemed suitable for neurosurgical intervention.
Treatment
Patients in the control arm were treated with 20 Gy in five daily fractions, beginning on the day of simulation. Patients in the experimental arm were treated with a single 10 Gy fraction, delivered on the day of simulation. The radiotherapy fields were defined to include the anatomic area of spinal cord compression with a suitable margin, typically one to two vertebrae above and below the level of compression. All patients were simulated (conventional/CT) and underwent accurate localization of the treatment area on the treatment unit. All patients were treated with a linear accelerator or cobalt unit. Field arrangement was at the discretion of the simulating physician. If a direct posterior field was indicated, prescription was at cord depth. This is defined as the depth of the posterior border of the vertebral body. The depth of the posterior border of the vertebral body was calculated from the diagnostic MRI images.
Randomization
Participants were randomly assigned following simple randomization procedures (computerized random numbers) to one of two treatment groups. The allocation sequence was concealed from the researcher enrolling and assessing participants in sequentially numbered, opaque, sealed envelopes. Envelopes were opened only after the enrolled participants completed all baseline assessments and it was time to allocate the intervention. Sealed randomization envelopes were prepared centrally prior to enrolling patients in the study in each individual participating center. These envelopes contained treatment assignments in random order. Each of the participating hospitals had their own series of sequential randomization envelopes, and the randomization arms were statistically pre-determined from a randomization master list. A sequentially numbered envelope was opened for each patient by the investigator/radiation therapist/research nurse once the patient had given signed informed consent. The study could not be blinded, as this is not practical in the case of a radiation study addressing a fractionation question.
Assessment
The evaluation scales used for symptom/functional evaluation were an in-house modified Tomita 3-point scale for the primary endpoint of mobility (1= ‘Unaided’, 2= ‘With walking aid’ and 3= ‘Bed-bound), a pain visual analog scale (VAS), and the EORTC QLQ–C30 questionnaire. The EORTC QLQ-C30 is a questionnaire developed to assess the QoL of cancer patients. It has well-established reliability and validity and is used in more than 3000 studies worldwide. The QLQ-C30 is composed of both multi-item scales and single-item measures. These include five functional scales, three symptom scales, a global health status/QoL scale and six single items. Questionnaires were answered prior to treatment and at 5 weeks, 3 months and every 3 months thereafter from completion of treatment. All patients were followed up until death. Except for the pretreatment questionaire, these questionnaires were completed over the telephone by the trial coordinator, a radiation therapist.
In a study of breast cancer or small-cell lung cancer patients, Osoba et al. found that the significance of changes in QLQ-C30 scores can be interpreted in terms of small, moderate, or large changes in QoL [Citation17]. For patients who indicated ‘a little’ change either for better or for worse, the mean change in scores was about 5–10; for ‘moderate’ change, about 10–20; and for ‘very much’ change, greater than 20. It is likely that the minimally important difference ranges from 5 to 10 points.
Statistical analysis
For EORTC QLQ-C30 scores, changes in scores over time for every patient were calculated by subtracting the results at baseline from those at the time of follow-up. Mean differences (with 95% confidence interval) in change between the groups were computed. A paired samples t-test was used to compare differences, from baseline to the 5-week follow-up, in the QLQ-C30 Summary score (excl. financial impact (FI) and global quality of life (QL)) [Citation18], the physical functioning scale and the pain score. For the QLQ-C30 summary score (excl. FI and QL) and the physical functioning scale, a high score represents a high QoL/healthy level of functioning, but a high pain score represents a high level of symptomatology/problems. One-way between-groups analyses of covariance (ANCOVA) were conducted to compare the effectiveness of the two different treatment schedules on the QLQ-C30 Summary score [Citation18] and on the pain control score assessed using a VAS. For these analyses, the independent variable was the radiation schedule the dependent variable was the score at 5 weeks post-treatment, and the covariate was the baseline score. Preliminary checks were conducted to ensure that there was no violation of the associated assumptions of normality, linearity, homogeneity of variances and homogeneity of regression slopes.
Categorical variables were analyzed using chi-square tests and continuous variables were analyzed using Kruskal–Wallis tests. All available data from eligible patients were included in the analyses. All statistical tests were two-sided and assessed for significance at the .05 level. Statistical analyses were carried out using IBM® SPSS® statistical software version 21 (IBM SPSS Statistics, Armonk, NY, USA). All analyses were performed according to the intention to treat principle but this did not differ from a per-protocol analysis because of the design of the trial (see ). The protocol required that there was to be no imputation of missing values.
Role of the funding source
Funding was received from St. Luke’s Institute of Cancer Research and the Health Research Board. The funding source had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. Cancer Trials Ireland (formerly ICORG), as the study sponsor, was involved in protocol development and approval, auditing the study, in the collection of data and in the decision to submit the article for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patient accrual
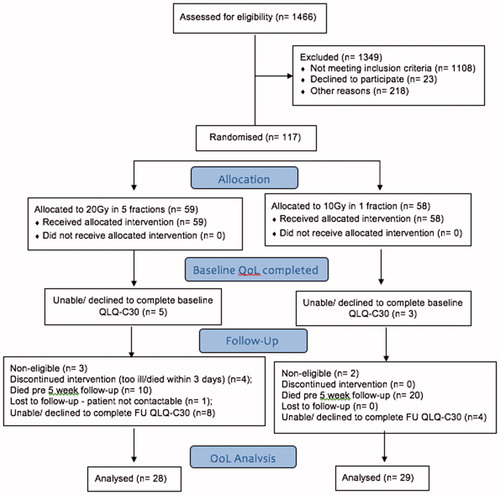
From January 2006 to April 2014, five Irish institutions accrued 117 patients. Five patients were found to be ineligible. All available data for eligible patients were included and analyzed. Eight patients were unable or declined to complete the QoL questionnaire at baseline. Five patients in the control group were too ill or died before the five fractions were delivered (one such patient had no baseline QoL completed). Thirty patients died before the 5-week follow-up. One patient in the control group was lost to follow up and another 12 were unable or declined to complete the QoL questionnaire due to weakness, tiredness, illness or an autonomous choice made by the patient. Consequently, data from 57 patients were available for this QoL analysis. is a participant flow diagram that includes the number who were randomly assigned, who received intended treatment, and who were analyzed for the QoL outcome in each group.
One hundred and four eligible patients completed the baseline QLQ-C30 questionnaire. Baseline demographic and clinical characteristics of these 104 patients are shown in grouped by those who were and were not analysed for the QoL outcome. Patients with prostate cancer had significantly better mean baseline QLQ-C30 Summary scores (excl. FI and QL) than patients with lung cancer or breast cancer (). Fifty six percent of patients with Lung cancer had ‘Very much’ pain compared to 41% of Breast cancer patients and 44% of Prostate cancer patients.
Table 1. Baseline demographic, clinical characteristics and QLQ-C30 scores.
Table 2. Baseline QLQ-C30 Summary scores (excl. FI and QL) by initial diagnosis (n = 104).
A one-way between-groups analysis of variance was conducted to explore the impact of baseline mobility on the baseline EORTC QLQ-C30 Physical functioning scale score (). There was a statistically significant difference in the Physical functioning score for the three mobility categories: F(2, 103) = 43.8, p < .0005. The actual difference between the groups was very large. The effect size, calculated using eta squared, was 0.46. Post-hoc comparisons using the Tukey HSD test indicated that the mean score for each group was statistically significantly different from each other group.
Patient characteristics
Baseline demographic and clinical characteristics of the 57 patients analyzed for the change in QoL for each treatment group are shown in . The main primary tumor sites were breast (34%) prostate (29%), and lung (7%). At baseline, 56% of patients had the ability to mobilize unaided (48% in the control arm and 63% in the experimental arm). Twenty percent could mobilize with the assistance of a walking aid while 24% were bed-bound. Patients were followed until death or for a median of 7 months (range: 1–103 months) from the end of RT.
Question 1: Is RT improving QoL?
A paired samples t-test (n = 51) showed clinically and statistically significant benefits of radiotherapy for the EORTC QLQ-C30 Summary score (excl. FI and QL) and pain score, from baseline (mean: 55.7 and 68.0, respectively) to the 5 week follow-up (mean: 67.3 and 36.9, respectively) t (50) = −4.6 and +5.1, respectively, p < .0005 in each case. There were no clinically or statistically significant benefits of radiotherapy for the physical functioning score or for the Global quality of life score from baseline (mean: 42.0 and 45.6, respectively) to the 5 week follow-up (mean: 44.0 and 51.0, respectively) t (50) = −0.5 and −1.3, respectively, p = .632 and .212, respectively.
shows the effects of the above four EORTC QLQ-C30 scores by treatment arm. The mean change from baseline to the 5-week follow up by treatment schedule is presented.
Table 3. Treatment effects on EORTC QLQ-C30 quality of life (QoL) outcomes (no comparison between arms).
For pain score, the analysis included all patients with a documented pain VAS score at 5 weeks. Overall EBRT – whatever the radiation schedule – led to a significant improvement of the pain with an improvement (decrease > 1 unit) and stabilization (±1) of the pain score in 57 and 14% of patients, respectively. The treatment intervention unfortunately did not prevent a worsening of the pain (increase > 1) in 29% of patients (). A one-way between-groups analysis of covariance compared the effectiveness of the two radiation schedules on patients’ VAS. After adjusting for pre-intervention scores, there was no significant difference between the two radiation schedules on 5-week VAS scores (p = .320).
Table 4. Visual analog scale pain score at week 5 post RT (n = 71).
Question 2: Is there a difference in QoL outcomes between fractionation schedules?
One-way between-groups analyses of covariance were conducted to compare the effectiveness of two different treatment schedules on the EORTC QLQ-C30 Summary score (excl. FI and QL) and the pain score. The independent variable was the treatment schedule and the dependent variable consisted of the Summary score and the pain score 5 weeks after treatment was completed. Patients’ scores pretreatment were used as the covariates in these analyses. Preliminary checks were conducted to ensure that there was no violation of the associated assumptions.
After adjusting for pre-intervention scores, there was no statistically significant difference in post-treatment Summary scores (excl. FI and QL), between the two RT schedules F(1, 48) = 0.03, p = .859, partial eta squared = 0.001. There was a statistically significant relationship between the pre-treatment and post-treatment summary scores (p = .002, partial eta squared = 0.186).
Similarly, after adjusting for pre-intervention scores, there was no statistically significant difference between the two RT schedules on post-treatment pain score F(1, 48) = 0.00, p = .985, partial eta squared= 0.000. In contrast to the Summary scores (excl. FI and QL), there was no statistically significant relationship between the pre-treatment and post-treatment pain scores (p = .785, partial eta squared = 0.002). Therefore, the statistically significant benefit of radiotherapy for the pain score, assessed by the t-test above, is no longer evident once the pre-intervention pain score is taken into account.
QoL data
On average, patients experienced an increase in QoL from baseline to the 5-week follow-up no matter what their initial diagnosis. Lung cancer patients experienced the greatest mean change in QoL but we must interpret this with caution as only four patients with lung malignancies were recruited to this study (the confidence interval of the mean does not support the greater effect in these patients). This change ranged from 2.4 to 39.2 points better than baseline ().
Table 5. EORTC QLQ-C30 Summary score change from baseline to 5-week follow-up by primary cancer.
A Kruskal–Wallis Test revealed a significantly significant difference in the physical functioning score across three different Mobility scores (Mobility score 1, n = 25; mobility score 2, n = 14; mobility score 3, n = 12), χ2 (2, n = 51) = 27.1, p < .0005. Those with mobility score 1 had the highest median PF score at week 5 (Md = 73), followed by those with mobility score 2 (Md = 47) and then by mobility score 3 (Md = 0).
3-Month follow-up
QoL data were available for 36 patients at the 3-month follow up. After adjusting for pre-intervention scores, there was no statistically significant difference between the two RT schedules on post-treatment Summary scores (excl. FI and QL) or Pain scores. Nor was there a statistically significant relationship between the pre-treatment and post-treatment pain scores. A statistically significant relationship between the pre-treatment and post-treatment Summary scores was not found (p = .075, partial eta squared = 0.093) ().
Table 6. Treatment effects on EORTC QLQ-C30 quality of life (QoL) outcomes 3 Months after treatment was completed.
All evaluable data included.
Subgroup analysis
An unplanned exploratory analysis was conducted on all those randomized excluding those non-eligible and those lost to follow-up (n = 111), to characterize patients with extremely short life expectancy, e.g., dying prior to week 5 assessment, who might not benefit from RT at all. In the control arm, four patients discontinued intervention (too ill/died within 3 d) and another 10 died before their 5-week follow-up appointment. In the treatment arm, 20 patients died prior to this point. Primary malignancy (Chi-square test: χ2 (3, n = 111) = 18.6, p < .0005, phi = 0.41), and initial mobility status (Chi-square test, χ2 (2, n = 111) = 13.1, p = .001, phi = 0.34) were found to be associated with dying before the 5-week follow-up. Sixty-eight percent of lung cancer patients and 14% of breast cancer patients died before their follow up at 5 weeks. Fifty-one percent of bed-bound patients and 15% of patients who could walk unaided died before their 5-week follow up.
EORTC QLQ-C30 questionnaire and compliance
Eight out of the 112 eligible patients did not complete the QLQ-C30 questionnaire at baseline. Of the other 104, only four patients had missing data for some of the items (0.2% of patient data were missing). For these four patients, at least half of the items from the scale were answered, except for one patient who had not answered either of the items in the social functioning scale.
Fifty-seven patients (51% of eligible patients) completed the follow-up QLQ-C30, 51 at the 5-week follow-up and 6 at a later time point. Of the 51, only two patients had missing data for some of the items (0.26% were missing). For these two patients, at least half of the items from the scale were answered, except for one patient who had not answered either of the items in the role Functioning scale.
So, while missing forms were a problem for this patient population, compliance for item completion was high, with only 0.19 and 0.26% of responses missing for each of the QLQ-C30 scales at baseline and at follow-up, respectively.
Discussion
In the recent Cochrane review George et al. concluded that ambulant adults with metastatic extradural MSCC with stable spines and predicted survival of less than six months will probably benefit as much from one dose of radiation (8 Gy) as from two doses (16 Gy) or eight doses (30 Gy) [Citation19]. None of the studies in their review reported on satisfaction with care or QoL. Since the abstract of ICORG 05-03 was presented at ASTRO 2014 [Citation14], another two RCTs of radiotherapy schedules have been published. Both SCORE-2 and the SCORAD III compared a multifractionated schedule with fewer or single fraction and found no difference in primary end-point [Citation20,Citation21].
L’Espérance et al. carried out a review of the scientific literature published up to February 2011, examining only phase II and III trials that included assessment of neurologic function [Citation22] and identified 26 studies. The authors made a number of recommendations including that short-course radiotherapy are administered to patients with spinal cord compression and short life expectancy (Tokuhashi score 0–8). Accordingly, they recommended that long-course radiotherapy is administered to patients with inoperable spinal cord compression and good life expectancy (Tokuhashi score 9–15). Included in their analysis is an article by Aass et al. who set out to identify prognostic factors and prospectively evaluate daily life function and pain experience in hormone-resistant prostate cancer patients with spinal cord compression treated with radiotherapy [Citation23]. They found that radiotherapy might improve mobility, daily life activity and sphincter control in patients with metastatic spinal cord compression due to hormone-resistant prostate cancer and recommend that radiotherapy should, therefore, be considered in all patients irrespective of the neurological deficits.
Overall, MSCC carries a poor prognosis. Measurement of the success of palliative treatment is much more difficult than that of radical treatment where concrete endpoints, such as death can be used. The concept of QOL is complex, elusive and individual. Indeed QoL indicators at the end of life have shown that overly aggressive cancer treatment may be an indicator of poor-quality care [Citation24,Citation25].
Radiotherapy improved QoL but not physical functioning. The reason for this relates to the fact that QLQ-C30 includes five functional scales, three symptom scales, a global health status/QoL scale and six single items. Physical functioning scale is just one of these. Therefore, if a patient has no improvement in physical functioning but has less pain, they will possibly have improved QoL. Similarly, the primary endpoint showed stability of mobility score but very little improvement. This is possible because QoL is subjective and mobility score is not. It is also possible that a patient could have an improvement in physical functioning that is not related to mobility as defined in this study. The primary endpoint mobility score improved for eight patients. Two patients improved from score 3 to score 1; five patients improved from score 3 to score 2 and one patient improved from score 2 to score 1.
The challenge of conducting trials in the studied population is the high attrition rate related to early death reported in other trials from 7 [Citation26] to 24% at 4 weeks [Citation20], 51% [Citation21] at 8 weeks post- treatment and 35% at 5 weeks in the present trial. As demonstrated by the published evidence, patient selection is crucial, and refinement of currently available individualized prognosis prediction tools is necessary to allow personalized management plans. The observed high early death rate – within the first 2 months after completion of radiotherapy – supports the hypothesis that some patients will not benefit from primary radiotherapy.
In the literature, it has been found that prognostic factors include initial mobility status, type of primary malignancy, other bone or visceral metastases, interval between tumor diagnosis and MSCC, duration of motor deficits, Karnofsky score and primary tumor (rapid versus intermediate versus slow) [Citation27]. Like Rades et al., we found that primary malignancy and initial mobility status were found to be associated with dying before the 5-week follow-up [Citation28]. We also tested for Gender (p = .133).
A potential source of bias in this study was the overall amount of missing EORTC QLQ-C30 forms over the course of the assessment period, with more missing data in the control arm. This problem tempers our ability to generalize these effects to future patients. It is possible that the EORTC QLQ-C15-PAL [Citation29], an abbreviated 15-item version of the EORTC QLQ-C30 developed for palliative care would have been a better choice of instrument for this patient group. Additionally, patient self-reporting has its limitations. Compliance may be poor, and some patients may record new pain at a different site, not acknowledging that the pain in the treated site has improved/disappeared. However, these factors are likely to be the same in each arm of the trial.
Conclusion
Primary radiotherapy significantly improves QoL as assessed by the EORTC QLQ-C30 summary score (excl. FI and QL). After adjusting for pre-intervention scores, there was no statistically significant difference between the two RT schedules on post-treatment Summary scores. Tumor site, mobility at diagnosis, muscle weakness and baseline EORTC QLQ-C30 summary score (excl. FI and QL) should be considered when offering radiotherapy to patients with MSCC and it should be recognized that certain patients with particularly poor life expectancies may not benefit at all from EBRT. For most, an effective treatment with low burden for the patient would be desirable. A single fraction schedule should be considered for this group of patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Spiller W. Rapidly progressive paralysis associated with carcinoma. Arch Neurol Psychiatry. 1925;13:471.
- Loblaw DAJ. Emergency treatment of malignant extradural spinal cord compression: an evidence-based guideline. J Clin Oncol. 1998;16:1613–1624.
- Gilbert R. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3:40–51.
- Helweg-Larsen S. Symptoms and signs in metastatic spinal cord compression: a study of progression from first symptom until diagnosis in 153 patients. Eur J Cancer. 1994;30A:396–398.
- Posner J. Neurologic complications of cancer. Philadelphia (PA): FA Davis Company; 1995. p. 111–142.
- Patchell RA, Regine WF, Payne R, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648.
- Hoskin P, Bhana R. Metastatic spinal cord compression: radiotherapy outcome and dose fractionation. Radiother Oncol. 2003;68:175–180.
- Maranzano E, Rossi R, De Angelis V, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol. 2005;23:3358–3365.
- Chow E. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25:1423–1436.
- Loblaw DA, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol). 2003;15:211–217.
- Rades D. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24:3388–3393.
- Rades D. Role of radiotherapy in the treatment of motor dysfunction due to metastatic spinal cord compression: comparison of three different fractionation schedules. Int J Radiat Oncol Biol Phys. 2002;54:1160–1164.
- Rades D. A prospective evaluation of two radiotherapy schedules with 10 versus 20 fractions for the treatment of metastatic spina cord compression. Cancer. 2004;101:2687–2692.
- Thirion P, O’Sullivan L, Clayton-Lea A, et al. ICORG 05-03: prospective randomized non-Inferiority phase 3 trial comparing two radiation schedules in malignant spinal cord compression not proceeding with surgical decompression. Int J Radiat Oncol. 2014;90:1263–1264.
- Aaronson N, Ahmedzai S, Bergman B, et al. The European Organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376.
- Bjordal K, de Graeff A, Fayers P. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (The EORTC QLQ-H&N35). Eur J Cancer. 2000;36:213–219.
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144.
- Giesinger J, Kieffer J, Fayers P, et al. EORTC Quality of Life Group. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J Clin Epidemiol. 2015;69:79–88.
- George R, Jeba J, Ramkumar G, et al. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev. 2015;9:CD006716.
- Rades D, Segedin B, Conde-Moreno AJ, et al. Radiotherapy with 4 Gy x 5 versus 3 Gy x 10 for metastatic epidural spinal cord compression: final results of the SCORE-2 trial (ARO 2009/01). J Clin Oncol. 2016;34:597–602.
- Hoskin P, Misra V, Hopkins K, et al. SCORAD III: randomized noninferiority phase III trial of single-dose radiotherapy (RT) compared to multifraction RT in patients (pts) with metastatic spinal canal compression (SCC). J Clin Oncol. 2017;35(18_suppl):LBA10004.
- L’Espérance S, Vincent F, Gaudreault M, et al. Treatment of metastatic spinal cord compression: cepo review and clinical recommendations. Curr Oncol. 2012;19:3478–3490.
- Aass N, Fosså SD. Pre- and post-treatment daily life function in patients with hormone resistant prostate carcinoma treated with radiotherapy for spinal cord compression. Radiother Oncol. 2005;74:259–265.
- Earle C. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21:1133–1138.
- Earle C. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2005;26:3860–3866.
- Maranzano E, Trippa F, Casale M, et al. 8Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol. 2009;93:174–179.
- Leithner A, Radl R, Gruber G. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J. 2008;17:1488–1495.
- Rades D, Douglas S, Veninga T, et al. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer. 2010;116:3670–3673.
- Groenvold M, Petersen M, Aaronson N, et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer. 2006;42:55–64.