Abstract
Background: Hearing loss is a well-known long-term effect after cisplatin-based chemotherapy (CBCT) in testicular cancer survivors (TCS), but longitudinal data are sparse. We evaluate hearing loss and the impact of age in TCS treated with CBCT in this longitudinal study.
Material and methods: Forty-six TCS treated with CBCT 1980–1994 with audiograms (0.25–8 kHz) pre-chemotherapy (PRE) and at a follow-up survey (SURV) after median 10 years were included (cases). Audiograms at SURV from 46 age-matched TCS without CBCT were included as controls. Linear regression was performed to evaluate predictors for change in the hearing threshold level (HTL) from PRE to SURV. Two definitions of a audiogram-defined hearing loss was applied if: (1) mean HTL for both ears exceeded 20 dB at any frequency 0.25–8 kHz (American Speech-Language-Hearing Association (ASHA) definition) and (2) average HTL for the frequencies 0.5, 1, 2 and 4 kHz exceeded 20 dB (WHO-M4 definition). Self-reported hearing impairment (SURV) was assessed by a questionnaire.
Results: Age and cisplatin dose was significantly associated with a greater change in HTL for the frequencies 2–8 kHz. For the 8 kHz frequency, each 100 mg increase in cumulative cisplatin dose was associated with a deterioration of 3.6 dB (95% CI 1.8–5.3, p < .001). The prevalence of hearing loss (ASHA) among cases was 33% PRE, 70% at SURV and 65% among controls at SURV (cases vs. controls, p = .66). According to M4, the prevalence of hearing loss among cases was 6.5% PRE, 13% at SURV and 2.2% among controls at SURV (cases vs. controls, p = .049). Twenty-nine percent of cases, and 33% of controls (p = .70) reported hearing impairment at SURV.
Conclusion: Cisplatin is associated with a hearing loss particularly at higher frequencies. Age appear to be an important factor for hearing loss regardless of treatment. The ASHA definition overestimates the hearing problem.
Introduction
Germ-cell testicular cancer (TC) is the most common malignancy among young men, and the incidence is rising [Citation1]. After the introduction of cisplatin in the late 1970s, most men with metastatic disease are cured and are expected to live for another 30–50 years after treatment [Citation2]. Thus, there are a growing number of long-term TC survivors (TCS) after treatment with cisplatin-based chemotherapy (CBCT).
Ototoxicity is a typical side effect from cisplatin treatment [Citation3] and is a consequence of irreversible damage to the outer hair cells that begins in the basal region of the cochlea [Citation4]. This damage results in tinnitus and hearing loss particularly at high frequencies, and may affect lower frequencies if apical parts of the cochlea are affected. Although ototoxicity may be a small price to pay for achieving cure in contrast to more serious late effects as cardiovascular disease and second cancers [Citation5], clinical hearing loss or tinnitus may have a considerable negative impact on the survivor’s daily functioning.
Previous publications addressing ototoxicity in cisplatin-treated TCS have limitations, including the lack of a validated questionnaire to address self-reported hearing [Citation6,Citation7], audiogram testing limited to a few frequencies [Citation8,Citation9] and inclusion of patients treated with carboplatin [Citation9]. Most previous reports had a cross-sectional design not taking baseline hearing function or age into account, and different definitions of a hearing loss were applied [Citation3,Citation6,Citation8,Citation9]. The most comprehensive report on hearing function among TCS after CBCT was recently published by Frisina et al. [Citation3]. The authors noted that among 488 men treated with CBCT, 80% had a hearing loss according to the ASHA criteria, of whom 18% had a severe to profound hearing loss. In agreement with the accompanying editorial comment [Citation10], we question whether the ASHA criteria are adequate for the identification of a clinical significant hearing loss, and to what degree CBCT contributes to the age-related hearing loss. The aims of the present longitudinal study were to evaluate (1) hearing threshold levels (HTLs) in audiograms pretreatment, during chemotherapy and at follow-up survey, (2) audiogram-defined hearing loss according to different criteria and (3) self-reported hearing impairment and tinnitus at survey in 46 men treated for TC with CBCT.
Material and methods
Study patients
All Norwegian long-term survivors (≥5 years) of unilateral germ-cell TC treated 1980–1994, aged 18–75 years, were invited to participate in a national follow-up survey. This survey was performed 1998–2002 at five university hospitals, and consisted of a mailed questionnaire and an outpatient clinical examination including blood pressure measurements [Citation11]. Audiometry was optional at the survey, though finally evaluating only the 4 kHz frequency. Of 1814 eligible survivors, 1462 (81%) men participated in this survey ().
Figure 1. A flow diagram illustrating how participants in the present study were included. CBCT: cisplatin-based chemotherapy.
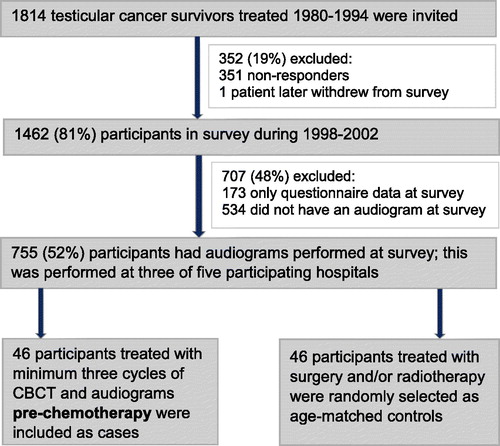
Pre-chemotherapy audiograms were not routinely obtained at the treating hospitals during the treatment period, but were occasionally obtained at two of the five participating hospitals. The present report is based on data from 46 participants (cases) who received minimum three cycles of CBCT and in whom audiograms (0.25–8 kHz) pre-chemotherapy and at survey were available. In addition, audiograms and questionnaires at survey from 46 age-matched TCS without CBCT were included as controls (). The study was approved by the Committee for Medical Research Ethics, South Health region. Informed consent was obtained from all individual participants included in the study.
Treatment principles, 1980–1994
Treatment principles have been described previously [Citation8]. CBCT was in 1980 introduced into the treatment of Norwegian patients with metastatic TC. With increasing clinical experience and based on available literature, the numbers of administered CBCT cycles decreased during the subsequent 15 years. However, all men with metastatic disease received minimum three cycles of CBCT. During 1990–1994, stage I non-seminomas with high-risk features received three cycles of CBCT [Citation12].
Chemotherapy consisted of cisplatin in combination with bleomycin and vinblastine (CVB) or more often, etoposide (BEP) [Citation13]. Cisplatin was generally administered at doses of 20 mg/m2 per day over five days. However, two patients in the present study received dose-intensive cisplatin treatment with 60 mg/m2 per day applied over three days (BEP-60) [Citation14].
Assessments
Overall 46 cisplatin-treated TCS with pure-tone audiograms (0.25–8 kHz) obtained for both ears before chemotherapy (PRE) and at follow-up survey (SURV) were included in this study. Among these, 29 men had additional audiograms performed during chemotherapy at the beginning of the last chemotherapy cycle (POST). The hearing threshold level (HTL) at each frequency was assessed as dB HL (hearing level). Of the 46 men, 11 men had additional extended high-frequency (EHF) audiograms at PRE and at SURV, where frequencies 9–14 kHz were assessed. According to convention, hearing thresholds in the high-frequency range were reported as dB SPL (sound pressure level) [Citation15].
Definition of hearing loss
ASHA has defined hearing loss as an HTL at any frequency exceeding 20 dB for either ear [Citation3,Citation16]. In this study, the mean HTL for both ears exceeding 20 dB at any frequency 0.25–8 kHz was classified as a hearing loss. We have also applied the World Health Organization (WHO) definition of a hearing loss, where a hearing loss is identified when the average HTL for the frequencies 0.5, 1, 2 and 4 kHz exceeds 20 dB, the so-called M4 definition [Citation17,Citation18].
At survey, the participants completed a 219-item questionnaire, including demographics, smoking habits, medication and the Scale for Chemotherapy-Induced Neurotoxicity (SCIN) questionnaire [Citation19]. In the present study, we have included the items addressing tinnitus ‘are you troubled by ringing in your ears?’ and impaired hearing ‘are you troubled by impaired hearing?’ from the SCIN questionnaire. For both questions, the following responses were possible: ‘not at all’, ‘a little’, ‘quite a bit’ and ‘very much’. When the responses were dichotomized, ‘a little’, ‘quite a bit’ and ‘very much’ indicated prevalent tinnitus or impaired hearing, vs. those without such symptoms [Citation3].
Statistics
Descriptive characteristics are presented with median (interquartile range or range) and frequency (percent). All reported hearing thresholds are means of the left and right ear. Differences between independent groups were tested using Wilcoxon rank sum test or chi-square test. Change over time in HTL for each frequency was calculated as (POST–PRE) or (SURV–PRE). The threshold values for each frequency at each time point were not normally distributed, but change in HTL was normally distributed. Since the hearing thresholds are strongly affected by age [Citation18], only crude change over the short time period in HTL (POST–PRE) was tested using Wilcoxon signed rank test. Age-adjusted logistic regression was used to evaluate possible associations between the dependent variables (tinnitus, audiogram-defined hearing loss or self-reported hearing impairment), and the independent variables cisplatin dose, hypertension and smoking in TCS treated with CBCT [Citation3].
Associations between change in HTL as the dependent variable and baseline HTL, age at follow-up, and cumulative cisplatin dose as independent variables were assessed using multivariable linear regression models. The regression coefficient β indicates the change in the dependent variable per unit increase in the variable of interest. Model assumptions were verified using residual analyses. All p values are two-tailed, with statistical significance set at p < .05.The data were analyzed using the SPSS 23.0 package (SPSS Inc., Chicago IL, USA).
Results
Patient characteristics
Median age at diagnosis was 29 years (range 21–54) and median observation time was 9.7 years (range 5–17) for the 46 cisplatin-treated TCS included in this study (). The majority of participants had non-seminomatous histology (72%), BEP was most frequently applied (83%) and the median number of CBCT cycles was 4 (range 3–8). Median age at follow-up was 39 years (range 27–62) for both 46 TCS treated with CBCT (cases) and 46 men without CBCT (controls).
Table 1. Baseline characteristics and patient-reported outcomes at survey for 46 TCS treated with CBCT with audiograms before CBCT and at survey, a subgroup of 29 TCS with additional audiograms immediately after CBCT and 46 age-matched controls without CBCT.
PRE and SURV audiograms
displays for the 46 cases the significant crude increases of HTLs from PRE to SURV at the frequencies 2–8 kHz, and the HTLs for 46 controls at SURV. The median HTL at SURV was significantly greater among cases than controls for the frequencies 2 kHz (median 7.5 vs. 2.5 dB, p = .037) and 4 kHz (median 20 vs. 13.8 dB, p = .037), with no statistically significant differences otherwise.
Figure 2. HTL for conventional range audiograms (0.25–8 kHz) for 46 men pre-chemotherapy ![]()
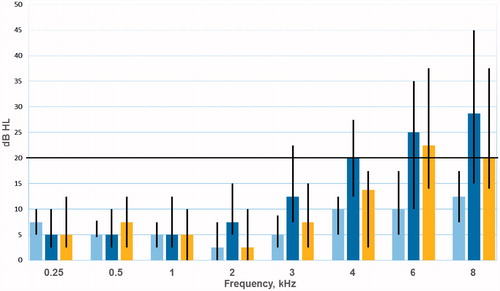
In the linear regression analyses for all 46 cases, age at survey and cumulative cisplatin dose were significantly associated with a greater change in HTL from PRE to SURV at the frequencies 2–8 kHz (), with gradually higher β values and lower p values with increasing frequencies. For the 8 kHz frequency, each 100 mg increase in cumulative cisplatin dose was associated with a deterioration of 3.6 dB (95% CI 1.8–5.3, p < .001).
Table 2. Linear regression with change in HTL from before chemotherapy (baseline) to survey as the dependent variableTable Footnotea.
PRE, POST and SURV audiograms
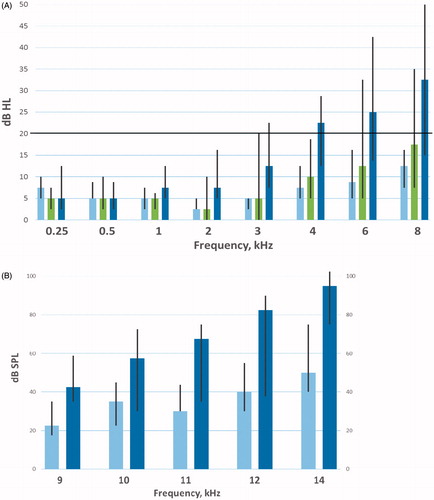
presents the median HTL in the frequency range 0.25–8 kHz for 29 men with audiograms at PRE, POST and SURV. The median HTL had significantly increased on POST compared with PRE audiograms for 6 kHz (12.5 dB vs. 8.75 dB, p = .007) and 8 kHz (17.5 dB vs. 12.5 dB, p = .026). Otherwise, no significant differences POST vs. PRE were detected. The median HTL for each frequency was <20 dB at PRE, SURV and POST audiograms except for the frequencies 4, 6 and 8 kHz at SURV (). For 11 men with EHF audiograms (9–14 kHz) at PRE and SURV, the median SPL threshold was >20 dB for all frequencies both PRE and SURV ().
Figure 3. (A) HTL (dB HL) for 29 men with conventional frequency audiograms (0.25–8 kHz) pre-chemotherapy ![]()
presents median changes in HTL and the number of men with ASHA-defined hearing loss for all 46 cases with PRE and SURV audiograms, and for 29 men with audiometric evaluations at PRE, POST and SURV (0.25–8 kHz). A hearing loss was rarely observed in the frequencies 0.25–1 kHz regardless of when the audiometries were performed. The number of men with a hearing loss increased with increasing frequency both POST and at SURV compared to PRE, but with a considerably larger number of men with a hearing loss at SURV than POST.
Table 3. Change over time in HTL and the number of men with ASHA-defined hearing loss at the different audiometric evaluations in the frequencies 0.25–8 kHz for all 46 cases (PRE and SURV), and for 29 men with all three audiometric evaluations (PRE, POST and SURV).
Audiogram-defined hearing loss
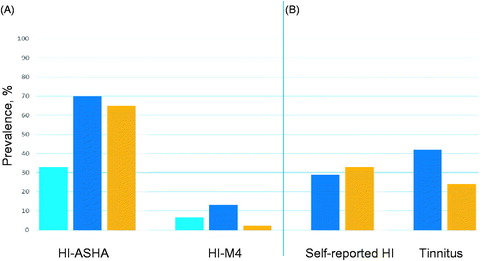
According to the ASHA criteria, as many as 15 of 46 cases (33%) had hearing loss before CBCT (). At SURV, the number of cases with hearing loss had increased to 32 (70%), this percentage not differing from that of the controls (70% vs. 65%, p = .66). Hearing loss according to the M4 definition was present in three cases (6.5%) before CBCT, and was significantly more often present in cases than controls at SURV (13% vs. 2.2%, p = .049). Hearing loss according to the M4 definition was associated with cisplatin dose (p = .018), but not with smoking status (p = 1.00), hypertension (p = .36) or tinnitus (p = .10) in cases at SURV.
Figure 4. (A) Hearing impairment (HI) according to the American Speech-Language-Hearing Association (HI-ASHA), the World Health Organization M4 criteria (HI-M4), (B) self-reported HI and self-reported tinnitus among cases (treated with chemotherapy) and controls. ![]()
Self-reported hearing impairment and tinnitus at survey
The prevalence of self-reported impaired hearing did not differ between cases and controls (29% vs. 33%, p = .70) (). Overall 13 cases (29%) reported some degree of hearing impairment, of which only three men (6.5%) reported more than ‘a little’ (). Self-reported hearing impairment was not associated with hearing loss according to the ASHA criteria (p = .22), but was associated with hearing loss according to the M4 criteria (p = .045) in cases. Hearing impairment was associated with cisplatin dose (p = .021) and tinnitus (p = .005), but not with smoking status (p = .93) or hypertension (p = .43) at SURV (age-adjusted logistic regression analyses) in cases.
Tinnitus was more frequently reported by cases than controls, but this difference was not statistically significant (42% vs. 24%, p = .06). Prevalent tinnitus was associated with increasing cisplatin dose (p = .012).
Discussion
To the best of our knowledge, this is the first longitudinal study in TCS evaluating hearing thresholds before and after CBCT that analyzes and visualizes the effect of CBCT on hearing loss and the concomitant effect of aging. We also show that CBCT leads to high-frequency HTL worsening early during chemotherapy, and a long-term increased HTL in the lower frequencies 2 and 4 kHz in comparison to controls. The M4 definition better than the ASHA criteria reflects self-reported hearing impairment.
At a median observation time of 10 years, we observed a gradually worsening in HTL with increasing frequencies, in line with previous publications [Citation3,Citation6,Citation7,Citation9]. In the Frisina paper, the frequencies 10 and 12 kHz had the greatest median threshold shifts at four years (median) post-treatment. It is of interest that in our study prior to chemotherapy, median EHF values (9–14 kHz) already exceeded the threshold (20 dB) for an ASHA-defined hearing loss. Although EHF audiometry is important in the identification of early changes in auditory function among children treated with CBCT [Citation15], it is of limited value in adults who receive CBCT as they have an existing age-related deterioration of hearing in higher frequencies even before initiation of treatment. To our knowledge, only one previous publication has presented hearing thresholds before and after CBCT for TC [Citation7]. The authors reported HTL shifts at long-term follow-up for the 2 and 8 kHz frequencies in comparison with the pretreatment situation, but their analyzes did not consider the effect of increasing age. CBCT seems to accelerate this age-related reduction of the hearing ability, with the possibility of premature presbycusis during longer follow-up.
The prevalence of an ASHA-defined hearing loss at survey at 70% in our cases is somewhat lower than the percentage presented by Frisina et al. at 80% [Citation3]. An obvious explanation is the inclusion of the 10 and 12 kHz frequencies in the Frisina study, while we have only included frequencies in the range 0.5–8 kHz. The ASHA definition of hearing loss overestimates the hearing problem since it does not separate between the different frequencies. The ASHA criteria might indicate ongoing or established ototoxicity, but most of the results cover higher frequencies and are of less clinical significance for the daily life of most patients. The prevalence of an audiogram-defined hearing loss [Citation16,Citation17] doubled between pretreatment and survey regardless of which definition we applied (ASHA: 33–70%, M4: 6.5–13%). However, only the M4 definition of a hearing loss was associated with self-reported hearing impairment in our study. In the present publication we chose to apply the ASHA definition enabling comparison with the large American series [Citation3]. We also used the M4 definition for hearing loss providing an instrument of higher clinical relevance. Several other grading systems for platinum-induced hearing loss are available [Citation20]. All of the published ototoxicity criteria have different limitations, for example, some of the grading systems are specific for children [Citation20], and we agree with a recent Cochrane review [Citation21] that there is a need for developing robust ototoxicity grading scales for cancer survivors.
Our prevalence of self-reported hearing impairment (29%) and tinnitus (42%) in TCS treated with CBCT at follow-up survey is identical with data presented by Frisina, who also applied the validated SCIN questionnaire [Citation3]. Tinnitus was more prevalent among cases than controls (42% vs. 24%), and was associated with cisplatin dose in our study. The prevalence of tinnitus increases with increasing age [Citation22], and although tinnitus is related to cochlear damage, also central nervous system mechanisms have been hypothesized to be involved in the pathogenesis of tinnitus [Citation23]. The lack of difference between cases and controls regarding the prevalence of self-reported hearing impairment at survey is surprising, since normal speech is considered to affect lower frequencies [Citation17] that are also moderately influenced by cisplatin according to our results.
In our study, the HTL was significantly greater for the frequencies 6 and 8 kHz during chemotherapy than before treatment, corroborating the data presented by Noszek et al. [Citation24], who reported that the deterioration in HTL for high frequencies was already present after one cycle of CBCT. The deterioration in hearing function in higher frequencies seems to begin immediately after cisplatin administration, and is probably the result of an acute injury to the outer hair cells in the cochlea [Citation4]. Our data support previous reports, showing that the long-term hearing loss in higher frequencies in TCS is strongly related to the cumulative cisplatin dose [Citation3,Citation6,Citation9]. Long-term hearing loss and tinnitus are also associated with the use of dose-intensive cisplatin treatment [Citation8]. Incomplete elimination of cisplatin after treatment results in measurable serum levels of platinum several years after administration [Citation25,Citation26]. Further, the long-term platinum levels were associated with tinnitus even after adjusting for the cisplatin dose in the publication by Sprauten et al. [Citation26]. The importance of platinum retention was highlighted in a recent publication which demonstrated particularly high long-term retention of cisplatin in the human cochlea [Citation27], which may contribute to ongoing damage of the hair cells several years after treatment with a risk of accelerated presbycusis.
The degree of cisplatin-induced hearing loss is associated with specific genetic variants, including glutathione S-transferase genotypes and variants of WFS1 in several studies [Citation11,Citation28–30]. It might be possible to identify individuals with a high risk of ciplatin-induced ototoxicity based on genotype in the future, but prospective and randomized studies are necessary. The use of protective agents during CBCT to avoid ototoxicity is an interesting principle [Citation4], although concerns could be raised regarding a possible interference with the therapeutic efficacy of cisplatin. Agents with promising results include amifostine, N-acetylcysteine, curcumin and antioxidant therapy including vitamin E [Citation31–34], but clinical trials are lacking or show ambiguous results, and no drugs can so far be recommended for clinical use.
To reduce cisplatin-induced ototoxicity, limiting the number of CBCT cycles as well as avoiding dose-intensive treatment would seem important. Avoidance of other ototoxic drugs such as aminoglycoside antibiotics [Citation4] during or shortly after cisplatin treatment is essential. Patients with TC who need treatment with cisplatin should be informed about the potential risk for high-frequency hearing loss, accelerated presbycusis and tinnitus. However, the most important factor for long-term worsening of the hearing function is increasing age, both in the general population [Citation18] and among TCS. We strongly advise doctors not to overestimate the risk of hearing loss associated with cisplatin when discussing this issue with patients.
The major strength of this study is the longitudinal design with audiometries pretreatment, before the last chemotherapy cycle and at follow-up survey. Other strengths include a long observation time, the inclusion of age-matched controls at survey and the assessment of self-reported hearing and tinnitus by using a validated questionnaire [Citation19]. Limitations include the lack of baseline audiograms and EHF audiograms for controls, a small number of patients, lack of information regarding noise exposure and occupation, and that patients were treated a long time ago with in part outdated CBCT regimens. However, the majority of included men received treatment with BEP, which has been the standard treatment for metastatic TC during the last 30 years [Citation2].
In conclusion, this study shows that cisplatin is associated with a moderate hearing loss, particularly at higher frequencies, but this risk should not preclude that patients are offered CBCT for metastatic TC. Aging is important for hearing loss in TCS regardless of treatment, but CBCT seems to increase the risk of developing accelerated presbycusis. The ASHA definition of hearing loss overestimates the hearing problem and should not be used as a clinical tool.
Acknowledgments
Thanks to project secretaries Vigdis Opperud and Siri Lothe. The study is a clinical study as part of the Norwegian Urological Cancer Group (NUCG) III project.
Disclosure statement
S.D. Fosså has received funding from Janssen Norway and Astellas Norway, and has an advisory role for Janssen Norway and Astellas Norway. There are no other author disclosures.
Additional information
Funding
References
- Le Cornet C, Lortet-Tieulent J, Forman D, et al. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur J Cancer. 2014;50:831–839.
- Feldman DR, Bosl GJ, Sheinfeld J, et al. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–684.
- Frisina RD, Wheeler HE, Fossa SD, et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol. 2016;34:2712–2720.
- Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken). 2012;295:1837–1850.
- Haugnes HS, Bosl GJ, Boer H, et al. Long-term and late effects of germ cell testicular cancer treatment and implications for follow-up. J Clin Oncol. 2012;30:3752–3763.
- Bokemeyer C, Berger CC, Hartmann JT, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–1362.
- Osanto S, Bukman A, Van HF, et al. Long-term effects of chemotherapy in patients with testicular cancer. J Clin Oncol. 1992; 10:574–579.
- Brydoy M, Oldenburg J, Klepp O, et al. Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst. 2009;101:1682–1695.
- Glendenning JL, Barbachano Y, Norman AR, et al. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer. 2010;116:2322–2331.
- Oldenburg J, Gietema JA. The sound of silence: a proxy for platinum toxicity. J Clin Oncol. 2016;34:2687–2689.
- Oldenburg J, Kraggerud SM, Cvancarova M, et al. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25:708–714.
- Swedish and Norwegian Testicular Cancer Group [Internet]. [cited 2017 Oct 12]. Available from: http://www.swenoteca.org/
- Williams SD, Birch R, Einhorn LH, et al. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316:1435–1440.
- Fossa SD, Saeter G, Aass N, et al. Management of patients with poor-prognosis nonseminomatous germ cell cancer. Oncology. 1990;47:234–240.
- Knight KR, Kraemer DF, Winter C, et al. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25:1190–1195.
- Clark JG. Uses and abuses of hearing loss classification. ASHA. 1981;23:493–500.
- World Health Organization: Future programme developments for prevention of deafness and hearing impairment [Internet], 1997. Available from http://apps.who.int/iris/bitstream/10665/63421/1/WHO_PDH_97.3.pdf
- Homans NC, Metselaar RM, Dingemanse JG, et al. Prevalence of age-related hearing loss, including sex differences, in older adults in a large cohort study. Laryngoscope. 2016;127:725–730.
- Oldenburg J, Fossa SD, Dahl AA. Scale for chemotherapy-induced long-term neurotoxicity (SCIN): psychometrics, validation, and findings in a large sample of testicular cancer survivors. Qual Life Res. 2006;15:791–800.
- Waissbluth S, Peleva E, Daniel SJ. Platinum-induced ototoxicity: a review of prevailing ototoxicity criteria. Eur Arch Otorhinolaryngol. 2017;274:1187–1196.
- van As JW, van den Berg H, van Dalen EC. Platinum-induced hearing loss after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;CD010181.
- Engdahl B, Krog NH, Kvestad E, et al. Occupation and the risk of bothersome tinnitus: results from a prospective cohort study (HUNT). BMJ Open. 2012;2:e000512.
- Saunders JC. The role of central nervous system plasticity in tinnitus. J Commun Disord. 2007;40:313–334.
- Noszek L, Budai B, Prekopp P, et al. Early ototoxic changes in patients with germ cell tumor after first cycle of cisplatin-based therapy. Laryngoscope. 2017;127:E277–E282.
- Hjelle LV, Bremnes RM, Gundersen PO, et al. Associations between long-term serum platinum and neurotoxicity and ototoxicity, endocrine gonadal function, and cardiovascular disease in testicular cancer survivors. Urol Oncol. 2016;34:487.e413–487.e420.
- Sprauten M, Darrah TH, Peterson DR, et al. Impact of long-term platinum concentrations on neuro- and ototoxicity in cisplatin-treated testicular cancer survivors. J Clin Oncol. 2012;30:300–307.
- Breglio AM, Rusheen AE, Shide ED, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. 2017;8:1654.
- Oldenburg J, Kraggerud SM, Brydoy M, et al. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5:70.
- Ross CJ, Katzov-Eckert H, Dube MP, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009;41: 345–1349.
- Wheeler HE, Gamazon ER, Frisina R, et al. Variants in WFS1 and other Mendelian deafness genes are associated with cisplatin-associated ototoxicity. Clin Cancer Res. 2017;23:3325–3333.
- Gurney JG, Bass JK, Onar-Thomas A, et al. Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma. Neuro Oncol. 2014;16:848–855.
- Fetoni AR, Paciello F, Mezzogori D, et al. Molecular targets for anticancer redox chemotherapy and cisplatin-induced ototoxicity: the role of curcumin on pSTAT3 and Nrf-2 signalling. Br J Cancer. 2015;113:1434–1444.
- Kalkanis JG, Whitworth C, Rybak LP. Vitamin E reduces cisplatin ototoxicity. Laryngoscope. 2004;114:538–542.
- Feghali JG, Liu W, Van De Water TR. L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope. 2001;111:1147–1155.
