Abstract
Background: Interleukin-2 (IL2)-based immunotherapy is curative for a small subset of patients with metastatic renal-cell carcinoma (mRCC). Preclinical data suggests that bevacizumab (BEV), a humanized anti-VEGF monoclonal antibody, has potential immunomodulatory effects by permitting efficient natural killer (NK) cell-mediated killing and by reverting immune suppression.
Patient and methods: We performed a randomized phase II study comparing IL2/IFN (interferon)/BEV with IL2/IFN in favourable/intermediate-risk mRCC patients. One hundred and eighteen patients received IFN 3 MIU subcutaneously (sc) daily and IL2 2.4 MIU/m2 sc twice daily, 5 days per week for two consecutive weeks every 28-day-cycle, for 9 months; or supplemented with BEV 10 mg/kg, every 2 weeks intravenously (iv) until progression, unacceptable toxicity, or 1 year following no evidence of disease (NED). Primary end point was progression-free survival (PFS).
Results: Baseline characteristics were well-balanced between the two arms; metastasis-free interval <1 year (75 versus 76%); prior nephrectomy (85 versus 86%); MSKCC favourable/intermediate-risk group (51/49 versus 52%/48%); three or more disease sites (41 versus 44%), respectively. The median PFS was 8.0 mo (95% CI, 4.2–11.9) with IL2/IFN/BEV and 8.1 mo (95% CI, 5.1–11.0) with IL2/IFN, p = .73. There was no difference in secondary endpoints, IL2/IFN/BEV versus IL2/IFN; median time-to-treatment failure (7.4 versus 5.6 mo, p = .54), response rate (44.1 versus 28.8%, p = .13), surgery of residual disease (17.0 versus 17.0%, p = 1.0), patients achieving NED (3.4 versus 8.5%, p = .44), and median overall survival (30.3 versus 34.1 mo, p = .39), respectively. TKI post progression was well-balanced (85 versus 78%). No new/unexpected toxicity was observed. Most common Grade 3/4 adverse events for IL2/IFN/BEV and IL2/IFN were fatigue (64 versus 61%), flu-like symptoms (37 versus 41%) and thrombosis (6.8 versus 18.6%, p = .01), respectively.
Conclusions: The addition of BEV to IL-2/IFN did not add efficacy in mRCC. (ClinicalTrials.gov, NCT01274273.).
Introduction
For decades, immunotherapy has been established therapy for the treatment of patients with mRCC. The cytokine IL2 was discovered in 1976 [Citation1], cloned in 1983 [Citation2] and approved in 1992 by the US Food and Drug Administration for the treatment of patients with mRCC. This drug is curative for a small subset of patients with mRCC [Citation3,Citation4]. Recently, the check-point inhibitor nivolumab demonstrated improved overall survival (OS) compared with contemporary standard therapy in second-line mRCC and received regulatory approval in 2015 [Citation5], but long-term follow-up data from responding patients are still pending.
The understanding of clear-cell RCC as a source of excess vascular endothelial growth factor (VEGF) production, due to inactivation of the von-Hipple Lindau tumor suppressor gene, has led to the development of anti-angiogenic therapy [Citation6]. Sustained inhibition of VEGF with BEV, a humanized anti-VEGF monoclonal antibody, results in the regression of existing tumour microvasculature and inhibition in the formation of new vasculature [Citation7,Citation8]. It may also revert tumor-associated immune suppression [Citation9], improve concomitant drug delivery into the tumour [Citation10,Citation11] and permit efficient natural killer (NK) cell-mediated killing [Citation12].
BEV was the first anti-VEGF therapy to demonstrate clinical benefit in mRCC, and other tumours [Citation13]. In mRCC patients, the addition of BEV to IFN compared with IFN alone produced statistically significant improvements in progression-free survival (PFS) and response rates [Citation14,Citation15]. BEV in combination with low-dose [Citation16] or high-dose IL2 [Citation17] was feasible in non-randomized trials, with response rate and PFS at least as high as reported previously for the single agents.
BEV, IFN and IL2 all have stimulatory effects on the immune response and these three agents may have complementary and synergistic effects when combined [Citation18]. We report the results from the Danish Renal Cancer Group (DaRenCa) study-1, a randomised phase II study to evaluate the efficacy and safety of BEV/IL2/IFN vs. IL2/IFN alone in patients with mRCC.
Patients and methods
Patients
DaRenCa-1 was an investigator-initiated, randomised, open-label phase 2 study. The study was run within the multidisciplinary national DaRenCa group with patients enrolled at two centres in Denmark. Eligibility criteria were patients ≥18 years, with locally advanced or metastatic RCC, a clear-cell histology component, no prior treatment, measurable disease per RECIST v.1.1 [Citation19], Memorial Sloan Kettering Cancer Center (MSKCC) favourable or intermediate risk [Citation20], Karnofsky Performance Status ≥70%, and adequate organ function (based on standard laboratory tests including hematology, serum chemistry, coagulation and urinanalysis). Exclusion criteria were brain metastases, spinal cord compression, uncontrolled hypertension, clinically significant cardiovascular or wound healing comorbidities, or corticosteroid requirement ≥10 mg/day. The study was approved by the Danish Medicines Agency, the Regional Research Ethics Committee, and the Danish Data Protection Agency; and adhered to the Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent. The study was registered at ClinicalTrials.gov, NCT01274273.
Patients were randomised 1:1 to receive IL2 and IFN-α or IL2 and IFN plus BEV. Randomisation was stratified by MSKCC risk group [Citation20] utilizing a stratified permuted blocks design. Study personnel did not have access to the master list. This was an open-label study to allow appropriate management of adverse events.
Treatments
All cytokines were administered subcutaneously (s.c.) over 4-week cycles for up to a maximum of 9 cycles (i.e., 9 months): IFN was given as a fixed dose, 3.0 MIU s.c. once daily for 5 days per week commencing with a priming-week of daily IFN. IL2 was given 2.4 MIU/m2 s.c. two times daily, 5 days per week, Weeks 1 and 2 every 4-week cycle. BEV at doses of 10 mg per kg of body weight was given every 2 weeks intravenously (i.v.) until disease progression, unacceptable toxicity, withdrawal of consent or a maximum of 1 year following obtaining no evidence of disease (NED).
Treatment interruptions, dose reductions or discontinuations were allowed to manage adverse events. In case of Grade 3 toxicity, with fatigue and fever as exceptions, therapy was delayed until toxicity was Grade 2 or less. For hepatic or renal Grade 3 toxicity, IL2 and IFN were dose reduced 50%. Intravenous (IV) fluid was allowed to prevent hypotension or renal toxicity. No dose reduction for BEV was permitted. In case of Grade 4 toxicity therapy was permanently discontinued. Crossover between treatment groups was not permitted. Physical examination, vital signs, laboratory assessments and urinalysis were conducted every 2 weeks for the first 4 weeks and then every 4 weeks thereafter. Adverse events were graded according to CTCAE v.3·0.CT were conducted at screening and every 12 weeks for the first 2 years and then every 6-months thereafter. Tumour response and progression were assessed by local radiologists according to Response Evaluation Criteria in Solid Tumours (RECIST) v.1·1 [Citation19]. Patients were not allowed to continue treatment if clinical benefit and/or biochemical improvements were seen in spite of radiological progression according to RECIST 1.1. Pseudoprogression was newer a challenge.
Endpoints
The primary endpoint was PFS, defined as the time from randomisation to date of progression per RECIST v.1·1 or death. Secondary endpoints were objective response rate (ORR), OS, time-to-treatment failure (TTF), tolerability, frequency of surgical resection of residual disease and frequency of NED. TTF was defined as time from randomisation to date of disease progression, death, withdrawal of treatment due to adverse events or laboratory abnormality, or withdrawn informed consent. Overall survival was defined as the time from randomisation to date of death from any cause, and ORR was defined as the proportion of patients experiencing best objective response as complete or partial response per RECIST 1·1.
Statistical analysis
The study was designed to provide adequate power for detecting a statistically significant difference in the primary endpoint of PFS. Assuming a median progression-free survival of 5 months in the IL2/IFN arm and 9 months in the IL2/IFN/BEV arm, corresponding to an 80% improvement in PFS, then 85 events were required to achieve overall 80% power of the log rank test at a two-sided overall 5% α-level. In order to see the required number of events within the planned study duration a total of 118 patients were required. Median duration of PFS, TTF and OS, corresponding 95% confidence intervals, were estimated by the Kaplan–Meier method. All analyses were conducted using SPSS® software (IBM Corporation, Armonk, NY, USA) v.20.
Results
Patient characteristics
From 26 October 2009 through 21 November 2014, at two Danish centres 118 patients were randomised to receive IL2/IFN/BEV (n = 59) or IL2/IFN (n = 59) and followed until study closure 31 May 2017. Preliminary data were presented at the American Society of Clinical Oncology (ASCO) 2016 Annual Meeting [Citation21]. Updated and final data as of 31 May 2017 are now presented. At the time of study closure, 113 patients had progressed, 78 patients had died, and 7 patients had NED. Median follow-up of patients alive was 64 months (range 28.5–88.1 months). Baseline characteristics were well-balanced between the two arms (); metastasis-free interval <1 year (75 versus 76%); prior nephrectomy (85 versus 86%); MSKCC favourable/intermediate-risk group (51/49 versus 52%/48%); three or more disease sites (41 versus 44%), and metastases to lungs (80 versus 83%) lymph nodes (63 versus 63%), and liver (14 versus 15%), respectively. The extent of bone metastases were higher in the IL2/IFN/BEV arm (27%) versus than in the IL2/IFN arm (15%), the difference was not statistically significant (p = .18).
Table 1. Demographics and baseline characteristics.
Treatment
Median duration of exposure was similar in the two groups: seven cycles (range, 0–9) in IL2/IFN/BEV and six cycles (range, 0–9) in the IL2/IFN arm. The most frequent reasons for early treatment discontinuation in the IL2/IFN/BEV versus IL2/IFN groups were disease progression (63 versus 49%) and adverse events (19 versus 15%), respectively.
Efficacy
There was no difference in the primary endpoint between IL2/IFN/BEV and IL2/IFN; the median PFS was 8.0 mo (95% CI, 4.2–11.9 mo) with IL2/IFN/BEV and 8.1 mo (95% CI, 5.1–11.0 mo) with IL2/IFN, p = .73 ().
Figure 1. Kaplan–Meier plot of (A) progression-free survival, (B) TTF and (C) OS. All 118 randomised patients were included in the analysis. The number of patients censored is summarized by interval. Black line: IL2/IFN/BEV, dotted line IL2/IFN.
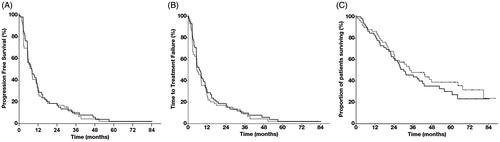
There was no difference in secondary endpoints between IL2/IFN/BEV and IL2/IFN; best ORR (44.1 versus 28.8%, p = .13) ( and ); surgery of residual disease (17.0 versus 17.0%, p = 1.0); and patients achieving NED (3.4 versus 8.5%, p = .44). Median TTF was 7.4 mo (95% CI 4.4–10.3 mo) with IL2/IFN/BEV versus 5.6 mo (95% CI, 2.6–8.5 mo) with IL2/IFN, p = .54 () and median OS was 30.3 mo (95% CI, 20.6–40.0 mo) with IL2/IFN/BEV versus 34.1 mo (95% CI, 19.9–48.2 mo) with IL2/IFN, p = .39 ().
Figure 2. Response characteristics. A swimmer plot depicting for each individual patient the time of response, duration of response, as well as surgery of residual disease.
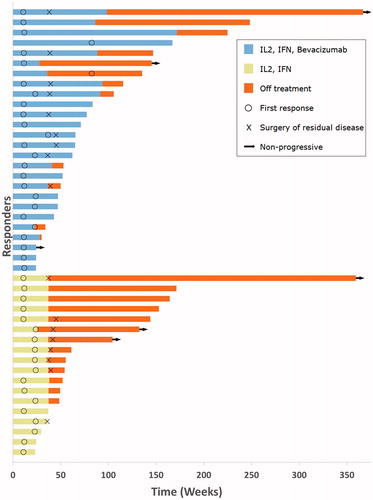
Table 2. Best overall objective response per RECIST 1.1.
Similar proportions of patients in the IL2/IFN/BEV and IL2/IFN groups had subsequent systemic anticancer therapy following study treatment discontinuation with no statistically significant difference between subgroups (85 versus 78%) ().
Exploratory analysis showed disparate outcomes for IL2/IFN/BEV versus IL2/IFN in MSKCC favourable and intermediate-risk groups. Patients with MSKCC favourable risk treated with IL2/IFN/BEV versus IL2/IFN had PFS 5.9 versus 8.5 mo, p = .18, TTF 5.5 versus 8.1 mo, p = .34 and OS 31.3 versus 64.6 mo, p = .018, respectively. Patients with MSKCC intermediate risk treated with IL2/IFN/BEV versus IL2/IFN had PFS 9.4 versus 2.9 mo, p = .17, TTF 8.5 versus 2.9 mo, p = .17 and OS 27.4 versus 18.9 mo, p = .32, respectively.
Toxicity
No new or unexpected toxicity was observed (). Most common Grade 3/4 adverse events for IL2/IFN/BEV and IL2/IFN were fatigue (64 versus 61%); flu-like symptoms (37 versus 41%); dehydration (25 versus 25%); hypertension (25 versus 3%, p = .08) and thrombosis (7 versus 19%, p = .01), respectively.
Table 3. Adverse events.
Discussion
This is the first report of combined VEGF blockade with BEV and IL2 plus IFN immunotherapy in patients with mRCC. The DaRenCa-1 study did not meet the primary endpoint of PFS. The randomised phase II study demonstrated the addition of BEV to IL2/IFN did not add efficacy. The safety profile of BEV was consistent with prior experience. Disparate outcomes were observed in the MSKCC favourable and intermediate-risk groups; the differences were not likely due to a difference in subsequent therapies that were evenly distributed, but may be due to small study number, chance, or unknown biologic differences between MSKCC favourable and intermediate-risk groups.
Previous investigations in mRCC patients of combination therapy with BEV and IFN versus IFN monotherapy resulted in a significantly longer PFS (8.5 versus 5.2 months) and RR (25.5 versus 13.1%) in the CALGB 90206 trial [Citation15]. The AVOREN phase III trial, demonstrated similar improvements in PFS (10.2 versus 5.4 months) and RR (31 versus 13%) [Citation14]. In both trials, however, BEV/IFN therapy did not improve OS statistically significant [Citation22,Citation23]. Our data are in line with these results, but did not add efficacy further, despite the addition of IL2. Our data are also in line with the non-randomized experiences with low-dose and high-dose IL2 in combination with BEV [Citation16,Citation17]; BEV in combination with high-dose IL2 did not increase durable responses [Citation17], as also seen in our study. With the extended follow-up of median 64 months, most patients eventually progressed with this low-dose IL2 regimen. At study closure, 7 patients (6%) had NED.
BEV has been used in combination with various agents for first-line treatment in clear-cell mRCC, without adding efficacy. The temsirolimus/BEV combination versus IFN/BEV was not superior in the INTORACT study [Citation24] or in the TORAVA study, where excess toxicity of the temsirolimus/BEV combination resulted in ∼50% of patients had to stop treatment [Citation25]. The efficacy of everolimus/BEV and IFN/BEV was similar in the RECORD 2 study [Citation26]. PFS was similar between BEV monotherapy (7.5 months) and BEV/temsirolimus, BEV/sorafenib, or BEV/IFN in the four-arm BEST trial [Citation27]. The combination of BEV and sunitinib resulted in excess toxicity and the combination was not considered feasible for further development [Citation28,Citation29]. Randomized phase III data for the combination of BEV and check-point inhibitors are pending.
IL2 has consistently resulted in durable complete responses and cure in a small subset of ∼5% of patients [Citation3,Citation30]. However, the toxicity associated with the drug has hindered the wide-use of IL2 and restricted its use to experienced centres. IL2 has been used in combination with various agents for first-line treatment in mRCC, without adding efficacy. IL2/histamine did not add efficacy compared with IL2 alone [Citation31]. IL2 alone, or combined with IFN, compared with the hormone drug medroxyprogesterone resulted in similar OS in intermediate-prognosis mRCC patients [Citation32]. Combination therapy of IL2/IFN and 5-fluorouracil (5-FU) chemotherapy did not improve efficacy compared with IFN alone [Citation33]. IL2/sorafenib resulted in similar survival rates as sorafenib alone [Citation34]. In essence, single agent BEV and single agent IL2 have demonstrated clinical efficacy in patients with mRCC, but attempts to improve efficacy, in terms of improved OS, by combination therapy with other drugs, have so far failed, including our study.
Toxicities did not exceed those expected from each agent alone. The most common adverse events were typical of those observed with these drugs including fatigue, flu-like symptoms, diarrhoea, nausea, decreased appetite, and hypertension [Citation6]. The lower incidence of thrombosis in the BEV arm is surprising, and was probably obtained by chance; it emphasizes, however, that the combination therapy was safe. Averse events were managed with supportive care in both treatment groups.
Subsequent anticancer therapy was balanced between treatment groups after study treatment discontinuation. Therefore, these factors are deemed unlikely to have biased the OS results towards one treatment group. In addition, treatment crossover was not allowed after determination of the primary endpoint of PFS enabling robust assessment of OS. Our data emphasizes that IL2 therapy does not compromise subsequent therapy, or clinical benefit, from subsequent therapy.
Eligibility criterion for the present study was treatment naïve mRCC patients that were not candidates for surgery. The tumour regression following systemic therapy enabling surgery of residual disease in 17.0% of patients should be noted, resulting in clinical meaningful benefit in a substantial part of patients. These benchmarks data provide perspective when interpreting the results from the next generation of immunotherapies, the check-point inhibitors. Identification of patients who will benefit from immunotherapy is crucial. This study was accompanied by sampling of blood, tumour and imaging biomarkers for predictive studies, which will be published separately. Functional imaging may represent a tool for selecting patients that will benefit, or not benefit, from therapy [Citation35].
In conclusion, the addition of BEV, an inhibitor of VEGF, to IL2-based immunotherapy was not associated with improvements in PFS, OS, TTF, ORR, surgery of residual disease, or patients with NED. This combination cannot be recommended for treatment in patients with mRCC.
Acknowledgments
We thank the patients, their families, the investigators and site staff, and the study teams who participated in the trial. FD received a research grant from Denmark Region Midt Research Fund.
This work was published as a poster in its preliminary form at the American Society of Clinical Oncology (ASCO) 2016 Annual Meeting, Chicago, IL, USA; 2 June 2016.
Disclosure statement
No potential conflict of interest was reported by the authors.
Roche provided BEV. Roche and Novartis did not have access to data.
Additional information
Funding
References
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008.
- Taniguchi T, Matsui H, Fujita T, et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983;302:305–310.
- Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301.
- Donskov F. Interleukin-2 based immunotherapy in patients with metastatic renal cell carcinoma. Dan Med Bull. 2007;54:249–265.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813.
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–366.
- Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997; 57:4593–4599.
- Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006; 116:2610–2621.
- Johnson BF, Clay TM, Hobeika AC, et al. Vascular endothelial growth factor and immunosuppression in cancer: current knowledge and potential for new therapy. Expert Opin Biol Ther. 2007;7:449–460.
- Wildiers H, Guetens G, De Boeck G, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003;88:1979–1986.
- Tong RT, Boucher Y, Kozin SV, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736.
- Markasz L, Stuber G, Vanherberghen B, et al. Effect of frequently used chemotherapeutic drugs on the cytotoxic activity of human natural killer cells. Mol Cancer Ther. 2007;6:644–654.
- Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434.
- Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370: 2103–2111.
- Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. JCO. 2008;26: 5422–5428.
- Garcia JA, Mekhail T, Elson P, et al. Clinical and immunomodulatory effects of bevacizumab and low-dose interleukin-2 in patients with metastatic renal cell carcinoma: results from a phase II trial. BJU Int. 2011;107:562–570.
- Dandamudi UB, Ghebremichael M, Sosman JA, et al. A phase II study of bevacizumab and high-dose interleukin-2 in patients with metastatic renal cell carcinoma: a Cytokine Working Group (CWG) study. J Immunother. 2013;36:490–495.
- Fricke I, Mirza N, Dupont J, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–4848.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247.
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. JCO. 1999;17:2530–2540.
- Donskov F, Jensen NV, Smidt-Hansen T, et al. A randomized phase II trial of interleukin-2/interferon-α plus bevacizumab versus interleukin-2/interferon-α in metastatic renal cell carcinoma (mRCC): results from the Danish Renal Cancer Group (DARENCA) study 1. J Clin Oncol. 2016;34 (Suppl.15): 4563.
- Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. JCO. 2010;28:2137–2143.
- Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. JCO. 2010;28:2144–2150.
- Rini BI, Bellmunt J, Clancy J, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. JCO. 2014;32:752–759.
- Negrier S, Gravis G, Perol D, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol. 2011;12:673–680.
- Ravaud A, Barrios CH, Alekseev B, et al. RECORD-2: phase II randomized study of everolimus and bevacizumab versus interferon alpha-2a and bevacizumab as first-line therapy in patients with metastatic renal cell carcinoma. Ann Oncol. 2015;26: 1378–1384.
- Flaherty KT, Manola JB, Pins M, et al. BEST: a randomized phase II study of vascular endothelial growth factor, RAF kinase, and mammalian target of rapamycin combination targeted therapy with bevacizumab, sorafenib, and temsirolimus in advanced renal cell carcinoma–a trial of the ECOG-ACRIN Cancer Research Group (E2804). JCO. 2015;33:2384–2391.
- Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–1439.
- Bruce JY, Kolesar JM, Hammers H, et al. A phase I pharmacodynamic trial of sequential sunitinib with bevacizumab in patients with renal cell carcinoma and other advanced solid malignancies. Cancer Chemother Pharmacol. 2014;73:485–493.
- Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. JCO. 1995;13:688–696.
- Donskov F, Middleton M, Fode K, et al. Two randomised phase II trials of subcutaneous interleukin-2 and histamine dihydrochloride in patients with metastatic renal cell carcinoma. Br J Cancer. 2005;93:757–762.
- Negrier S, Perol D, Ravaud A, et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer 2007;110:2468–2477.
- Gore ME, Griffin CL, Hancock B, et al. Interferon alfa-2a versus combination therapy with interferon alfa-2a, interleukin-2, and fluorouracil in patients with untreated metastatic renal cell carcinoma (MRC RE04/EORTC GU 30012): an open-label randomised trial. Lancet. 2010;375:641–648.
- Procopio G, Verzoni E, Bracarda S, et al. Overall survival for sorafenib plus interleukin-2 compared with sorafenib alone in metastatic renal cell carcinoma (mRCC): final results of the ROSORC trial. Ann Oncol. 2013;24:2967–2971.
- Mains JR, Donskov F, Pedersen EM, et al. Dynamic contrast-enhanced computed tomography-derived blood volume and blood flow correlate with patient outcome in metastatic renal cell carcinoma. Invest Radiol. 2017;52:103–110.
