Abstract
Background: Sphincter-sparing radiotherapy or chemoradiation are standard treatments for patients with anal cancer. The ultimate treatment goal is full recovery from anal cancer with preserved anorectal function. Unfortunately, long-term survivors often suffer from severe anorectal symptoms. The aim of the present study was to characterize changes in anorectal physiology after radiotherapy for anal cancer.
Method: We included 13 patients (10 women, age 63.4 ± 1.9) treated with radiotherapy or chemoradiation for anal cancer and 14 healthy volunteers (9 women, age 61.4 ± 1.5). Symptoms were assessed with scores for fecal incontinence and low anterior resection syndrome. Anorectal physiology was examined with anorectal manometry and the Functional Lumen Imaging Probe.
Results: Patients had a median Wexner fecal incontinence score of 5 (0–13) and a median LARS score of 29 (0–39). Compared to healthy volunteers, patients had lower mean (±SE) anal -resting (38 ± 5 vs. 71 ± 6, p < .001) and -squeeze pressures (76 ± 11 vs. 165 ± 15, p < .001). Patients also had lower anal yield pressure (15.5 ± 1.3 mmHg vs. 28.0 ± 2.0 mmHg, p < .001), higher distensibility, and lower resistance to flow (reduced resistance ratio of the anal canal during distension, q = 5.09, p < .001). No differences were found in median (range) rectal volumes at first sensation (70.5 (15–131) vs. 57 (18–132) ml, p > .4), urge (103 (54-176) vs. 90 (32-212), p > .6) or maximum tolerable volume (173 (86–413) vs. 119.5 (54–269) ml, p > .10).
Conclusion: Patients treated with radiotherapy or chemoradiation for anal cancer have low anal resting and squeeze pressures as well as reduced resistance to distension and flow.
Introduction
The incidence of squamous cell carcinomas in the anal canal (anal cancer, AC) has increased significantly during the last decades. Incidence rates have reached 1 per 100,000 person-years among men and 3 per 100,000 person-years among women [Citation1]. Sphincter-sparing radiotherapy (RT) or chemoradiation (CRT) have become the standard treatments for AC patients. The goal of treatment is full recovery from AC with preserved anorectal function. Hence, colostomy-free survival is a commonly used measure of clinical success.
Several large studies of various regimes of RT and/or CRT for AC have reported 5-year survival rates of 52–73% [Citation2–12]. Unfortunately, long-term survivors often suffer from severe anorectal dysfunction [Citation13–18]. In a study from our unit in Aarhus, Denmark, 31% of patients treated for AC suffered from incontinence to solid stools, 54% had incontinence to liquid stools, 79% had incontinence to gas at least once per month, and 56% had daily episodes of urgency to defecate [Citation14]. Radiotherapy may not only affect the anal canal. Radiation-induced rectal hypersensitivity can cause urge and fecal incontinence but remains to be studied in detail after treatment for AC. Unfortunately, the pathophysiology behind symptoms caused by RT or CRT remains poorly understood.
Previous studies on late (≥3 months) anorectal toxicity after radiotherapy in AC patients found decreased anal resting pressure and squeeze pressure [Citation16–18]. However, the competence of sphincters is better characterized by their dynamic resistance to distension (distensibility) than by their pressure profile [Citation19–23]. The functional lumen imaging probe (FLIP) allows dynamic and segmental examination of distensibility properties of the anal canal [Citation24–26]. The FLIP technique has previously been employed for studies of geometric and mechanical properties of the anal canal in patients with idiopathic fecal incontinence or with fibrotic degeneration of the internal anal sphincter caused by systemic sclerosis [Citation22,Citation23,Citation27–29].
We hypothesized that AC patients treated with RT or CRT will have decreased resistance to distension (increased distensibility) of the anal canal and experience rectal hypersensitivity. Thus, the aim of the present study was to characterize anal sphincter function and rectal sensitivity in AC patients treated with RT or CRT.
Material and methods
Subjects
We studied 13 AC patients (10 female) with a mean age of 63.4 ± 1.9 years and 14 healthy volunteers (9 female) with a mean age of 61.4 ± 1.5 years. Patients were consecutively recruited among those seen for routine control at the Department of Surgery, Aarhus University Hospital, Denmark. One patient had a T1 tumor, seven patients had T2 tumors, three patients had T3 tumors and two patients had T4 tumors. Nine patients had no regional lymph node metastases (N0), three had metastases in perirectal lymph node(s) (N1) and one had metastases in unilateral internal iliac and/or inguinal lymph node(s) (N2). None of the patients had distant metastases (M0).
All patients had been treated with RT or CRT at least 18 months prior to inclusion and were without recurrence. Mean time after treatment was 4.6 ± 0.3 years. All 13 patients had received three-dimensional conformal external beam radiotherapy with 64 Gy to the tumor site (Clinical Target Volume #1) and 51.2 Gy to the internal, external iliacal and inguinal lymph nodes (Clinical Target Volume #2) in 30 fractions with simultaneous integrated boost technique. Ten patients had received chemotherapy, including eight treated with cisplatin, 5-fluorouracil and ifosfamide, one treated with cisplatin and one treated with Taxol/Xeloda. Two patients with a T2N0 tumor and one patient with a T1N0 tumor were treated solely with RT as described above.
Data from the healthy volunteers, except for two men, have been part of a previous publication [Citation28]. Exclusion criteria for the healthy volunteers were fecal incontinence (gas incontinence was accepted), prior colorectal or pelvic surgery, other colorectal or pelvic disease or active use of medication known to interfere with GI function.
The study was conducted in conformity with the Declaration of Helsinki after approval by the local ethics committee (reference number 1-10-72-189-15).
Questionnaires
Both groups were evaluated using the Wexner fecal incontinence score [Citation30] The Wexner score consists of five questions (q) (incontinence for either solid stool (q1), liquid stool (q2), or gas (q3), pad usage (q4) and lifestyle impact (q5)) where each is assigned 0–4 points depending on frequency (never to daily). Hence, the global score ranges from 0 to 20.
The patients were further characterized using the Low Anterior Resection Syndrome (LARS) Score [Citation31]. The LARS score is a symptom-based scoring system for bowel dysfunction after low anterior resection with or without preoperative radiotherapy for rectal cancer [Citation32]. The score consists of five independent questions characterizing frequency, urgency, incontinence and clustering that are weighed and added up to a total score between 0 and 42 points. The LARS score allows categorization of patients into three groups: no LARS (0–20 points), minor LARS (21–29 points) and major LARS (30–42 points).
Experimental protocol
Both groups were evaluated using standard anal manometry and anal FLIP. Subjects were examined after two hours fasting and with an empty bladder. An enema (Microlax, McNeil, Birkerød, Denmark) was administered at least 15 minutes prior to the investigation, ensuring that the rectum was empty. All examinations were done with the subject in the left lateral position. Standard anal manometry and rectal bag volume distension (Menuet, Dantec medicals, Bristol, UK) was performed in accordance with a previous publication from our unit [Citation33].
Functional lumen imaging probe (FLIP)
The EndoFLIP® System (Crospon Ltd, Galway, UK) has previously been described in detail [Citation19,Citation27–29]. It is based on the impedance planimetry technique where luminal cross-sectional areas (CSA) of narrowing zones in hollow organs such as the anal canal are determined using a 12-cm-long noncompliant bag containing electrically conductive fluid. The electrical impedance is measured between 16 pairs of detection electrodes each placed 5 mm apart [Citation34]. In the present study, the FLIP procedure was initiated after 15 minutes of rest following the rectal distension protocol. The bag was filled at a rate of 40 mL/min and the bag had a maximum diameter of 25 mm. The pressure within the bag is determined by a solid-state pressure transducer. Pressure and CSA are sampled at 10 Hz and displayed directly on the monitor. Air was removed from the FLIP catheter and the intra-bag pressure set to zero before the FLIP procedure.
The FLIP catheter was inserted until only two detection electrodes were visible outside the anal verge. During the experiments, the catheter was held in position manually by one of the investigators. Initially, the bag was filled twice to 10 ml to ensure proper unfolding before the resting pressure were measured for 30 seconds. Afterwards, ramp distensions were done to 20, 30, 40, and 50 mL. Each ramp distension was performed at rest and followed by 30 seconds of iso-volumetric recording, also at rest, before the subject was asked to squeeze for 10 seconds. The investigator holding the catheter checked the position of the FLIP catheter visually during and after each squeeze.
Data analysis
Recordings from the FLIP were exported to a customized MATLAB subroutine (The Mathworks, Natick, MA, USA) for analysis of distension properties.
Spatio-temporal maps of the FLIP recordings
The diameters along the anal canal were shown as color-coded topography by interpolation of each channels diameter change over time as previously described [Citation22,Citation28]. The diameters were shown as single pixels by spatial orientation (y-axis) and time (x-axis). Finally, the bag pressure and volume were overlaid on the diameter map to ease interpretation of data.
Analysis of anal canal geometric properties
As described in previous studies, the pressure and the diameters along specific locations of the anal canal were analyzed at: (a) the initial state, (b) during rest, (c) during squeeze, and (d) during filling of the FLIP bag [Citation22]. The proximal and distal limits of the anal canal were determined from its initial state as previously described [Citation22,Citation27–29]. The geometric configuration of the anal canal was determined for the entire data recording. The diameters at the proximal, the middle and the distal sections of the anal canal were used for further analysis.
Distensibility and yield pressure of the anal canal
The distensibility of the anal canal was determined from the pressure-diameter diagrams obtained from the volume distension to 50 ml. The yield pressure was defined as the pressure when the middle part of the anal canal dilated to 110% of the initial diameter (size at volume 0 ml) followed by continuous increase in diameter during the inflation [Citation22]. Thus, the yield pressure expresses the pressure when the most resistant (middle) part of the sphincter begins to distend (CSA starts to increase).
Flow resistance of the anal canal
During the FLIP distension, the flow resistance of the anal canal (ACR) was defined from the anal canal length (ACL), the middle part diameter of the anal canal (ACMD) and the dynamic viscosity of the inflated bag (DV), as [Citation35]:
ACL was calculated as the distance between the proximal and distal anal canal location. Subjects were only included in this part of the analysis if we could clearly identify the two locations. The DV was assumed to be constant for all tests and hence the ACR/DV ratio (ACRR) was calculated as the measure of flow resistance of the anal canal as an expression of the anal sphincter resting tone when exposed to increasing volumes (10-50 mL).
Statistical analysis
Our primary endpoint was the anal yield pressure. Secondary endpoints were anal flow resistance and distensibility, as well as rectal sensitivity. Descriptive data are given as mean ± SEM or median (range) where appropriate. Two-way repeated measures ANOVA analysis was used for comparison of the diameter and pressure changes. The Tukey test was used for post hoc analysis. Squeezing and resting state pressure changes, as well as the resistance ratio at the resting state were compared between the two groups with factor 1: patients versus healthy volunteers, and factor 2: volumes. Diameters at the resting state in the middle and proximal anal canal were compared using factor 1: volumes, and factor 2: location within the anal canal. For both locations, the diameter changes at rest were compared between patients and healthy volunteers with factors 1: patients versus healthy volunteers and factor 2: volumes. Student t-test was used for comparison of the yield pressure, and Mann-Whitney Rank Sum Test was used for comparing the resistance ratio at pressure 40 mmHg during ramp distension when the volume increased from 0 to 50ml. Differences were considered statistically significant when p < .05. Pearson correlation coefficient was used to examine associations between pressures and symptom scores. All statistical analyzes were done using the software package Sigma Plot 11.0 (SPSS Inc. Chicago, IL).
Results
Clinical and demographical data are presented in .
Table 1. Demographic and clinical data on 14 healthy volunteers and 13 patients treated with radiotherapy for anal cancer. Data are reported in mean ± SE or median (range).
Bowel symptoms
The patients scored significantly higher on the Wexner fecal incontinence score compared to healthy volunteers. Patients had a median LARS score of 29 (range 0–39), which corresponds to minor LARS. Three patients had no symptoms of LARS (no LARS; 0–20 points) with a median score of 7 (range 0–10), four had minor LARS (21–29 points) with a median score of 29 (range 27–29) and six had major LARS (30–42) with a median score of 34.5 (range 32–39).
Standard anorectal manometry
Anal resting and squeeze pressures measured by conventional anal manometry were significantly lower in AC patients than in healthy volunteers (). No significant difference in rectal sensibility was found between the groups (). We found no association between Wexner fecal incontinence score and resting pressure (r = −0.31, p > .2), squeeze pressure (r = −0.06, p > .8) and yield pressure (r = 0.31, p > .3) nor between LARS score and resting pressure (r = −0.51, p > .1), squeeze pressure (r = −0.47, p > .1) and yield pressure (r = 0.53, p > .1).
Functional lumen imaging probe
The FLIP procedures were all performed without discomfort to the subjects. The spatio-temporal diameter maps during distension showed initial opening of the proximal anal canal followed by opening of the distal anal canal. The middle anal canal had the greatest resistance to distension, usually only yielding at bag volumes 40 and 50 ml (). In the resting state, all diameters along the anal canal in healthy and patients increased during distension (). The diameter of the proximal anal canal was the largest, followed by the distal and then the middle anal canal (q > 5.5, p < .05). Within each of the three locations there were no differences between patients and healthy subjects ().
Figure 1. Spatio-temporal diameter map of the anal canal from a patient with anal cancer (a) and a healthy volunteer (b). The changing colors from blue to red illustrate increasing diameter. Each column, depicts the configuration of the anal canal at a specific time and each row depicts change in diameter over time at a particular point along the FLIP probe. The vertical axis on the left represents the length of the anal canal and the right side represents the pressure (thin white line) and volume (heavy black dot line). (c) & (d), the configuration of the anal canal in (a) and (b) at the initial state with the visually defined proximal and distal limits and the computed points representing the proximal, middle and distal parts of the anal canal.
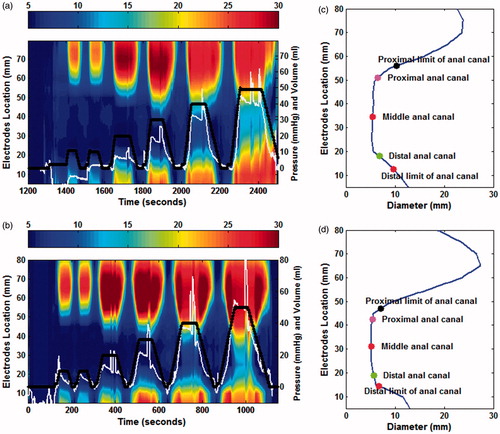
Figure 2. Averaged diameters and pressure of the anal canal in patients treated for anal cancer and healthy volunteers (HVs). (a) The diameter change of the middle (MP), proximal (PPC) and distal (DPC) anal canal during distension; b & c), the anal canal pressure during resting and squeeze states; (d) The squeeze-induced pressure increase to the resting pressure. Data are given as mean ± SEM. Diameter changes of the distal anal canal could not be assessed in three patients and five healthy volunteers because of slight movement of the FLIP probe.
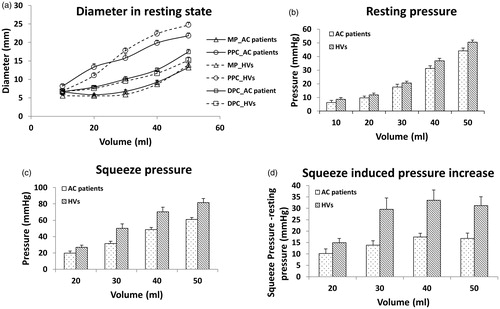
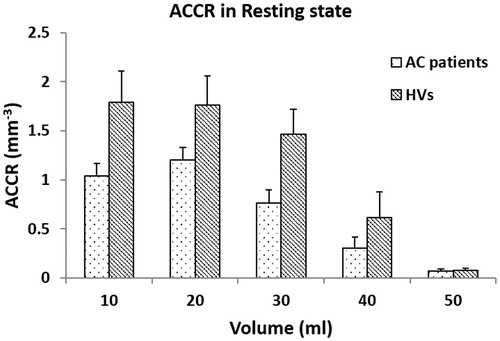
The FLIP recorded anal resting pressure, squeeze pressure and the squeeze-induced pressure increase were all significantly lower in patients than in healthy volunteers (q > 5.2, p < .001, ). The resistance ratio of the anal canal decreased with increasing distension pressure in both AC patients and healthy volunteers. The resistance ratio of AC patients was smaller for healthy volunteers at all distension volumes (, q = 5.09, p < .001).
Figure 3. The anal canal resistance ratio as function of the distension volume during the rest state.
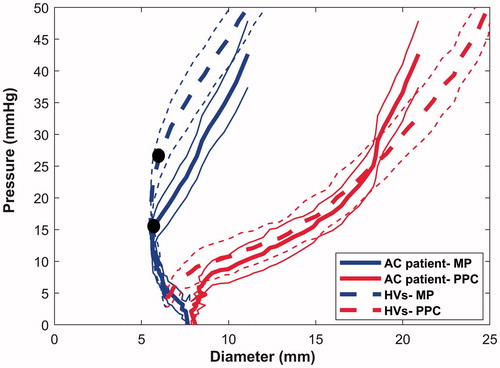
The averaged diameter-pressure relationship differed along the longitudinal axis of the anal canal with the proximal part being the softest (). The yield pressure of the anal canal was significantly lower in patients (15.5 ± 1.3 mmHg) compared to healthy volunteers (28.0 ± 1.9 mmHg) (t = −4.842, p < .001). The diameter-pressure curve for the middle part of the anal canal of patients was located to the right of that for the healthy volunteers. This also indicates that wall is softer in the patient group () at the same pressure level.
Figure 4. Pressure-diameter relationship of the anal canal. The averaged diameter and pressure relationship of the middle and proximal part of the anal canal during inflation up to 50ml is shown for patients treated for anal cancer (AC, heavy solid lines) and for healthy volunteers (HVs, heavy dotted lines). The middle part of the anal canal is less distensible than the proximal part. The curves from AC patients are located to the right of the corresponding curves from healthy controls, indicating a softer wall in patients. The two black markers indicate the yield pressure needed for opening of the middle part of the anal canal. The light solid and dotted lines are the 95 percent confidence intervals.
Discussion
The main findings of the present study were that patients treated with sphincter-sparing radiotherapy or chemoradiation for anal cancer had significantly reduced resistance to distension of the anal canal as well as reduced resistance to flow. With standard manometry, we confirmed previous findings of reduced anal resting and squeeze pressures after RT or CRT [Citation17,Citation18]. Differences between AC patients and healthy subjects were significant even though most patients only had minor symptoms.
Radiotheraphy for anal cancer
The anatomy of anal cancers makes treatment complicated. The anal canal is a caudal–posterior pelvic structure, whereas nodal spread from AC is both anteriorly to the inguinal lymph nodes and superiorly to the mesorectal, external and internal iliac lymph nodes. In order to cover these structures by conventional RT techniques, the field must include large parts of the bowel, the pelvic floor muscles, the bladder and genitalia. The patients in this study were treated with 3D conformal external beam RT, a technique that is now largely abandoned.
The main function of the anal canal is to maintain continence and to allow passage of stools at an appropriate time and place. The internal anal sphincter is the main contributor to the anal resting pressure. Somatic innervation submit the external anal sphincter and puborectalis muscle to voluntary control making them the main contributors to the anal squeeze pressure. Damage to any of the structures carries a risk of fecal incontinence or defecatory disturbances.
In most tissues, the most prominent pathological features of radiation are progressive fibrosis and vascular lesions, sometimes seen in the late phase reaction [Citation36,Citation37]. Reduced anal resting pressure is a common finding after pelvic radiotherapy, whereas reduced anal squeeze pressure seems less consistent [Citation38–41]. Further, increased amount of collagen induced by fibrosis is associated with fecal incontinence [Citation42].
Functional assessment of the anal canal after radiotherapy
Manometry is the standard method for assessment of sphincter function. It provides information on closure capacity. However, studies have indicated that distensibility more accurately reflects sphincter competence [Citation23]. The dynamic and segmental examination of distensibility properties of the anal canal allows us to study biomechanical changes induced by fibrosis in the anal canal.
Reduced anal resting pressure is commonly reported after pelvic RT, whereas the influence on anal squeeze pressure is more ambiguous [Citation38–41,Citation43]. We found that both anal resting and squeeze pressures were reduced when assessed with conventional anal manometry and with FLIP. Further, our study showed that AC patients treated with RT or CRT had reduced resistance to distension as well as reduced resistance to flow during distension in the anal canal. Findings were similar to previous data from patients with idiopathic fecal incontinence [Citation28], fecal incontinence of mixed etiologies [Citation23,Citation27] or fibrotic degeneration of the internal anal sphincter muscle in systemic sclerosis [Citation27]. This indicates that reduced resistance to distension in the anal canal is a contributing factor to symptoms related to late anorectal toxicity such as fecal incontinence.
Nerve damage is another well-known part of radiation toxicity. In the irradiated rectum of patients treated for prostate cancer, histologic examination has shown marked damage of the myenteric plexus which was associated with smooth muscle hypertrophy [Citation44,Citation45]. A study from our unit on rectal cancer patients who had received curative low anterior resection and developed major LARS showed that additional neoadjuvant therapy leads to severe impairment in neo-rectal sensitivity and blunted perception of urge to defecate, despite unaltered biomechanical wall properties [Citation43]. In this study, reduced resting anal pressure was also reported despite sparse radiation to the sphincter complex, suggesting damage to its neural innervation.
In the present study no differences in rectal sensation and compliance were found between AC patients and healthy volunteers. This may indicate that late anorectal toxicity after treatment of AC primarily affects the anal canal with a relative sparring of the rectum or that the rectum simply react differently to this radiation focus. However, evaluation of rectal sensorimotor function with standard balloon distension has significant methodological limitations, including large inter- and intraindividual variability and poor reproducibility related to the properties of the balloon [Citation46].
Whether the observed anal sphincter dysfunction is a result of structural or neurological injuries or a result of both remains to be determined. However, our findings of increased distensibility suggest a degenerative transformation of tissue, theoretically both muscle and nerve, as opposed to severe fibrosis. Examining the geometrical configuration of the anal canal during rest, we found a weakening of the hourglass-like shape in the anal canal of AC patients. This reflects reduced resistance in the middle part of the anal canal where the IAS is thickest and covered by the EAS. This may indicate damage of both the IAS and the EAS.
Limitations
FLIP is a promising method which is now commonly used for evaluation of the lower esophageal sphincter [Citation19]. Only few studies have evaluated anal sphincter function by means of the FLIP [Citation22,Citation27,Citation28]. The validity and limitations of the FLIP technique have been discussed in detail previously [Citation19,Citation27,Citation29]. FLIP is a relatively new method and the most appropriate way to analyze data is still not known. In our most recent anal FLIP publication, we defined yield pressure as the pressure when most narrow part of the anal canal was distended to 110% of its size at a volume of 0 ml [Citation22]. In the present study, there was no distension of the narrowest point during maximum filling of the FLIP bag in 2 out of 14 patients and 10 out of 14 healthy volunteers. This makes computation of biomechanical parameters difficult. Hence, we chose the opening pressure of the middle part of the anal canal as the yield pressure ().
A built-in limitation with FLIP is that the pressure is only determined at one location inside the bag. This poses no problem when the bag is distended and only consists of one compartment. However, if the bag is divided into two compartments, for example by a closed sphincter, the validity of pressure recordings is compromised. Another limitation of the present study is the small number of patients.
We studied 13 patients and 14 healthy volunteers with an almost perfect age- and sex-match. The number of included subjects may appear low. However, we obtained significant differences for the parameters hypothesized to be different. Other p values were higher than 0.2 (often higher than 0.4) indicating low risk for type-2 error. Hence, an even larger cohort would not have provided significance for those parameters.
We recruited AC patients who had finished their RT or CRT more than 18 months previously. As late anorectal toxicity may represent a dynamic process [Citation47], it could be interesting to repeat the study in 2-3years to reveal development over time. There is a possibility that patients with symptoms are more willing to participate in this type of study, causing us to overestimate the differences between AC patients and healthy volunteers by selection bias. Only two tumors were T4 tumors and we do not suspect that the alterations observed were caused by local ingrowth of the tumor.
We did not asses AC patients with Wexner nor LARS scores prior to treatment and it is possible, that some degree of symptoms were present even before treatment. For comparison, healthy volunteers were excluded at the presence of fecal incontinence, however, gas incontinence was accepted (median Wexner score of 1 (0-4)) rendering the selection representative of the age group compared.
Assessed with the Wexner fecal incontinence score, patients were not severely incontinent while most had symptoms of minor or major LARS. The latter was primarily due to the presence of urge to defecate and fecal clustering. The LARS score was develop from rectal cancer patient´s perception of the symptoms impact on their quality of life. To what degree the LARS score is applicable to AC patients remains to be determined [Citation14]. In previous studies among patients with anorectal toxicity after RT for prostate cancer, urge to defecate was associated with low anal pressures [Citation48,Citation49]. In the present study, reduced anal pressures were not associated with any of the symptom scores, most likely due to a type two error.
Our study population had a mean time since treatment of 4.6 ± 1.1 years . Treatment modalities have improved over the last decade and it is possible that patients treated more recently have suffered less collateral radiation damage than those treated 10 years ago.
Clinical implications
Our findings suggest that AC patients with anorectal toxicity after RT or RCT treatment suffer a generalized degeneration of pelvic floor tissue (muscle and nerves), mimicking that of fecal incontinence patients with decreased resistance to distension and flow. The quality of life in stoma-free survivors of anal cancer is relatively unaffected despite objective impairment of anorectal function [Citation17]. In the present study, patients were asked how much GI function affected their quality of life (not at all, a little, to some degree, a lot). Four patients answered a little and seven patients answered to some degree. One patient did not answer.
Treatment algorithms for late anorectal toxicity remain to be established. We have previously demonstrated that sacral nerve stimulation increases resistance to distension in IFI patients [Citation22]. The option of treating late anorectal toxicity with sacral nerve stimulation has not yet been explored. This leaves patients with conservative treatment including loperamide, dietary fiber, suppositories and transanal irrigation.
Conclusions
Our study shows that AC patients treated with RT or RCT have impaired anal sphincter function when assessed with standard manometry and with FLIP. Our data indicate that the main cause of symptoms is anal sphincter dysfunction rather than rectal hypersensitivity.
Disclosures statement
Hans Gregersen was one of the inventors of the FLIP technology and owns minority shares in Crospon Ltd. that manufactures FLIP. The other authors have nothing to disclose.
Acknowledgments
We thank nurses Gitte Sørensen, Margit Majgaard and Kirsten Haupt for their assistance in data collection and patients for participating.
Additional information
Funding
References
- Ministry of Health D. http://www.esundhed.dk/sundhedsregistre/CAR/CAR01/Sider/Tabel.aspx.
- Peiffert D, Tournier-Rangeard L, Gerard JP, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012;30:1941–1948.
- James R, Wan S, Glynne-Jones R, et al. A randomized trial of chemoradiation using mitomycin or cisplatin, with or without maintenance cisplatin/5FU in squamous cell carcinoma of the anus (ACT II). Jco. 2009;27:LBA4009.
- Nilsson PJ, Svensson C, Goldman S, et al. Epidermoid anal cancer: a review of a population-based series of 308 consecutive patients treated according to prospective protocols. Int J Radiat Oncol Biol Phys. 2005;61:92–102.
- Renehan AG, Saunders MP, Schofield PF, et al. Patterns of local disease failure and outcome after salvage surgery in patients with anal cancer. Br J Surg. 2005;92:605–614.
- Deniaud-Alexandre E, Touboul E, Tiret E, et al. Results of definitive irradiation in a series of 305 epidermoid carcinomas of the anal canal. Int J Radiat Oncol Biol Phys. 2003;56:1259–1273.
- Myerson RJ, Kong F, Birnbaum EH, et al. Radiation therapy for epidermoid carcinoma of the anal canal, clinical and treatment factors associated with outcome. Radiother Oncol. 2001;61:15–22.
- Peiffert D, Bey P, Pernot M, et al. Conservative treatment by irradiation of epidermoid cancers of the anal canal: prognostic factors of tumoral control and complications. Int J Radiat Oncol Biol Phys. 1997;37:313–324.
- Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054.
- Touboul E, Schlienger M, Buffat L, et al. Epidermoid carcinoma of the anal canal. Results of curative-intent radiation therapy in a series of 270 patients. Cancer. 1994;73:1569–1579.
- Wagner JP, Mahe MA, Romestaing P, et al. Radiation therapy in the conservative treatment of carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 1994;29:17–23.
- Papillon J, Montbarbon JF. Epidermoid carcinoma of the anal canal. A series of 276 cases. Dis Colon Rectum. 1987;30:324–333.
- Allal AS, Sprangers MA, Laurencet F, et al. Assessment of long-term quality of life in patients with anal carcinomas treated by radiotherapy with or without chemotherapy. Br J Cancer. 1999;80:1588–1594.
- Sunesen KG, Norgaard M, Lundby L, et al. Long-term anorectal, urinary and sexual dysfunction causing distress after radiotherapy for anal cancer: a Danish multicentre cross-sectional questionnaire study. Colorectal Dis. 2015;17:O230–O239.
- Bentzen AG, Guren MG, Vonen B, et al. Faecal incontinence after chemoradiotherapy in anal cancer survivors: long-term results of a national cohort. Radiother Oncol. 2013;108:55–60.
- Vordermark D, Sailer M, Flentje M, et al. Impaired sphincter function and good quality of life in anal carcinoma patients after radiotherapy: a paradox?. Front Radiat Ther Oncol. 2002;37:132–139.
- Vordermark D, Sailer M, Flentje M, et al. Curative-intent radiation therapy in anal carcinoma: quality of life and sphincter function. Radiother Oncol. 1999;52:239–243.
- Broens P, Van Limbergen E, Penninckx F, et al. Clinical and manometric effects of combined external beam irradiation and brachytherapy for anal cancer. Int J Colorectal Dis. 1998;13:68–72.
- Lottrup C, Gregersen H, Liao D, et al. Functional lumen imaging of the gastrointestinal tract. J Gastroenterol. 2015;50:1005–1016.
- Harris LD, Winans CS, Pope CE. 2nd. Determination of yield pressures: a method for measuring anal sphincter competence. Gastroenterology. 1966;50:754–760.
- Harris LD, Pope CE. 2nd. “Squeeze; vs. resistance: an evaluation of the mechanism of sphincter competence”. J Clin Invest. 1964;43:2272–2278.
- Haas S, Liao D, Gregersen H, Lundby L, Laurberg S, Krogh K. Increased yield pressure in the anal canal during sacral nerve stimulation: a pilot study with the functional lumen imaging probe. Neurogastroenterol Motil. 2017;29. DOI:10.1111/nmo.12929
- Gourcerol G, Granier S, Bridoux V, Menard JF, Ducrotte P, Leroi AM. Do endoflip assessments of anal sphincter distensibility provide more information on patients with fecal incontinence than high-resolution anal manometry? Neurogastroenterol Motil. 2016;28:399–409.
- Gregersen H, Orvar K, Christensen J. Biomechanical properties of duodenal wall and duodenal tone during phase I and phase II of the MMC. Am J Physiol. 1992;263:G795–G801.
- Gregersen H, Andersen MB. Impedance measuring system for quantification of cross-sectional area in the gastrointestinal tract. Med Biol Eng Comput. 1991;29:108–110.
- Gregersen H, Djurhuus JC. Impedance planimetry: a new approach to biomechanical intestinal wall properties. Dig Dis. 1991;9:332–340.
- Fynne L, Luft F, Gregersen H, et al. Distensibility of the anal canal in patients with systemic sclerosis: a study with the functional lumen imaging probe. Colorectal Dis. 2013;15:e40–e47.
- Sorensen G, Liao D, Lundby L, et al. Distensibility of the anal canal in patients with idiopathic fecal incontinence: a study with the Functional Lumen Imaging Probe. Neurogastroenterol Motil. 2014;26:255–263.
- Luft F, Fynne L, Gregersen H, et al. Functional luminal imaging probe: a new technique for dynamic evaluation of mechanical properties of the anal canal. Tech Coloproctol. 2012;16:451–457.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97.
- Emmertsen KJ, Laurberg S. Reconstructive techniques after rectal resection for rectal cancer. A survey of a Cochrane review. Ugeskr Laeger. 2009;171:55–58.
- Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255:922–928.
- Ryhammer AM, Laurberg S, Hermann AP. Test-retest repeatability of anorectal physiology tests in healthy volunteers. Dis Colon Rectum. 1997;40:287–292.
- Gregersen H. Biomechanical methods and analysis. In: Biomechanics of the gastrointestinal tract: new perspectives in motility research and diagnostic. London: Springer; 2003. p. 73–135.
- Farag A. The use of flow equation in functional coloproctology:a new theory in anorectal physiology. Pelviperineology. 2009;28:17–23.
- Fajardo LF. The unique physiology of endothelial cells and its implications in radiobiology. Front Radiat Ther Oncol. 1989;23:96–112.
- Haboubi NY, Schofield PF, Rowland PL. The light and electron microscopic features of early and late phase radiation-induced proctitis. Am J Gastroenterol. 1988;83:1140–1144.
- Lundby L, Krogh K, Jensen VJ, et al. Long-term anorectal dysfunction after postoperative radiotherapy for rectal cancer. Dis Colon Rectum. 2005;48:1343–1349.
- Pollack J, Holm T, Cedermark B, et al. Long-term effect of preoperative radiation therapy on anorectal function. Dis Colon Rectum. 2006;49:345–352.
- Ammann K, Kirchmayr W, Klaus A, et al. Impact of neoadjuvant chemoradiation on anal sphincter function in patients with carcinoma of the midrectum and low rectum. Arch Surg. 2003;138:257–261.
- Gervaz P, Rotholtz N, Pisano M, et al. Quantitative short-term study of anal sphincter function after chemoradiation for rectal cancer. Arch Surg. 2001;136:192–196.
- Yeoh EK, Holloway RH, Fraser RJ, et al. Pathophysiology and natural history of anorectal sequelae following radiation therapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2012;84:e593–e599.
- Bregendahl S, Emmertsen KJ, Fassov J, et al. Neorectal hyposensitivity after neoadjuvant therapy for rectal cancer. Radiother Oncol. 2013;108:331–336.
- Varma JS, Smith AN, Busuttil A. Function of the anal sphincters after chronic radiation injury. Gut. 1986;27:528–533.
- Varma JS, Smith AN, Busuttil A. Correlation of clinical and manometric abnormalities of rectal function following chronic radiation injury. Br J Surg. 1985;72:875–878.
- Krogh K, Ryhammer AM, Lundby L, et al. Comparison of methods used for measurement of rectal compliance. Dis Colon Rectum. 2001;44:199–206.
- Odrazka K, Dolezel M, Vanasek J, et al. Time course of late rectal toxicity after radiation therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:138–143.
- Smeenk RJ, Hopman WP, Hoffmann AL, et al. Differences in radiation dosimetry and anorectal function testing imply that anorectal symptoms may arise from different anatomic substrates. Int J Radiat Oncol Biol Phys. 2012;82:145–152.
- Krol R, Hopman WP, Smeenk RJ, et al. Increased rectal wall stiffness after prostate radiotherapy: relation with fecal urgency. Neurogastroenterol Motil. 2012;24:339–e166.
