Abstract
Purpose: Radiation-induced skin injury represents one of the most common side effects in breast cancer patients receiving adjuvant whole-breast radiotherapy. Numerous systemic and topical treatments have been studied in the prevention and management of radiation-induced skin injury without providing sustainable treatment strategies. While superficial barrier-forming skin products such as dressings are the standard of care in wound care management, their utilization as preventive treatment approach in radiotherapy has barely attracted attention.
Methods: In this prospective, intra-patient randomized study, Hydrofilm polyurethane film dressings were applied prophylactically to either the medial or lateral breast half of 62 patients with breast cancer undergoing adjuvant radiation therapy following breast conserving surgery. The breast half contralateral to the film dressing was concurrently treated with 5% urea lotion as control skin care. Maximum severity of radiation dermatitis was assessed using RTOG/EORTC toxicity scores, photospectrometric erythema measurements and patient-assessed modified RISRAS scale.
Results: In the Hydrofilm compartments, mean maximum RTOG/EORTC radiation dermatitis severity grades were significantly reduced from 1.33 to 0.35 and photospectrometric measurements showed significantly reduced erythema severity, as compared to the control compartments, with an overall response rate of 89.3%. Hydrofilm completely prevented moist desquamation and significantly reduced patients’ subjective experience of itching and pain.
Conclusion: The obtained results along with a favorable cost–benefit ratio and an easy and quick application suggest a prophylactic application of Hydrofilm in adjuvant radiotherapy of breast cancer patients to reduce or even prevent radiation dermatitis.
Introduction
Today’s therapy strategies mostly schedule an adjuvant radiotherapy in early and advanced breast cancer particularly following breast-preserving surgery [Citation1]. A frequent complication after radiotherapy is the acute radiation dermatitis with swelling, erythema, desquamation, burning and pain of the treated skin. These reactions may occur several weeks after radiotherapy commences and can last for weeks post-treatment. Especially in patients presenting with severe skin reactions, therapy interruptions are eventually indispensable which may compromise local tumor control [Citation2].
Several risk factors for acute radiation dermatitis have been identified – the risk is principally dependent on patient- and treatment-related factors: treatment-related factors are the applied radiotherapy technique (higher risk with conventional treatment planning as compared to IMRT), neoadjuvant or concomitant chemotherapy and re-irradiation. Patient-related factors are treatment site (most sensitive regions are the anterior of the neck, extremities, chest/breast, abdomen and face), older age, female sex, breast reconstructions and implants (inability to dissipate heat after reconstruction), connective tissue as well as radio-sensitivity disorders. Whether obesity, smoking or chronic sun exposure represent independent risk factors has not yet been definitively clarified [Citation2,Citation3].
Numerous clinical trials investigated preventive as well as management strategies for acute radiation dermatitis. While a few studies focused on the efficacy of systemic medications, most trials evaluated topical treatments. These covered a broad spectrum from washing behavior patterns, to the use of/omitting from deodorants to steroidal and varying non-steroidal topical treatments [Citation4–7].
Topical steroids are a well-validated treatment in the management of existing radiation dermatitis; data on their preventive potential, however, are inconsistent [Citation4,Citation8].
Most topical non-steroidal treatments aim at an improved skin moisture by rehydrating and/or cooling care creams. These are often derived from either cosmetic (e.g., hyaluronic acid) or natural medicine, partially with somewhat questionable anti-inflammatory properties. The obtained results were as variable as the broad range of drugs or mixtures used, and sometimes even contradictive [Citation4,Citation9,Citation10]. The majority of topical agents, however, were shown to be ineffective for reduction of incidence or severity of radiation-induced skin reactions.
Besides topical treatments, a few initial and promising results have been obtained with skin protection products in form of barrier-forming solutions, foam- or silicone-based dressings. The underlying concept assumes that the maintenance of the keratinized surface and protection of radiotherapy-affected basal stem-cell layer from superficial abrasion and friction may prevent skin damage to a large extent [Citation11–16].
In our radiotherapy department, Hydrofilm dressings have been used to protect patient positioning skin marks from fading. Hydrofilm is a transparent and quite thin polyurethrane film dressing, sticking to the skin surface by a hypoallergenic acrylic adhesive. The dressings are sterile, semi-permeable (water vapor permeability >1.100 g/m2/24 h), water- as well as bacteria-proof and can remain on the skin surface for up to two weeks without causing trauma on removal. Hydrofilm is approved as a primary dressing to cover post-operative and trauma wounds or as a secondary dressing for retention purposes [Citation17].
As we had observed reduced skin reactions under Hydrofilm, we initiated the following study to compare prophylactically applied Hydrofilm dressings with our standard skin care using moisturizing 5% urea lotion.
Material and methods
Participants
Between September 2016 and September 2017, all women receiving adjuvant whole-breast radiotherapy at our comprehensive academic cancer center were screened for recruitment. Inclusion criteria were: age >18 years, breast-preserving surgery for breast cancer and a fractionation regimen of 50 Gy in 25 fractions (fx) (patients younger than 65 years with pathology-confirmed invasive breast cancer received a sequential boost radiotherapy up to 66 Gy with 2 Gy/fx). Exclusion criteria were: neoadjuvant or concomitant chemotherapy, active smoking status, metastatic disease, previous radiation to the ipsilateral chest wall, breast reconstruction, active dermatitis, treatment with topical or oral corticosteroids, mastectomy, different fractionation regimens and patient refusal to participate.
Study methodology
This prospective, intra-patient controlled, single-center randomized clinical study was approved by the local Ethics Committee (186/2016). Written informed consent was obtained by all participants. Before treatment, the treated breast of each patient was divided into medial and lateral halves which were previously randomized by computer-generated randomization protocols to either Hydrofilm (Paul Hartmann AG, Heidenheim, Germany) or 5% urea lotion (Eucerin UreaRepair PLUS Lotion 5% Urea, Beiersdorf AG, Hamburg, Germany).
Eucerin UreaRepair PLUS lotion 5% urea is an oil-in-water emulsion, which is non-greasy, fragrance- and colorant-free and absorbed quickly. Dermatological and radiotherapeutic studies demonstrated that urea offers very good care efficacy and is well tolerated, even by extremely dry and diseased skin [Citation18,Citation19]; it is therefore recommended in the prevention of radiation dermatitis by several European medical guidelines and used as standard skin care in our institution [Citation20].
Due to the methodology of our study with patients acting as their own controls with visible film-dressings, blinding of both the radiation therapist and the patient was unfeasible.
Hydrofilm was applied immediately preceding the first radiation treatment on either the lateral or medial compartment of the irradiated breast by a radiation therapist, either in a seated or supine patient position, and replaced upon detachment or at least every two weeks. Special care was taken to achieve a smooth and unwrinkled attachment, especially in skin folds, to avoid patient discomfort or shear-stress.
Patients were given oral and written information to apply urea lotion twice a day to the irradiated control area adjacent to the Hydrofilm-covered compartment, starting at the first day of the radiation treatment. Compliance was checked by at least weekly visits, and patients were encouraged not to use any complementary topical treatment. Patients experiencing radiation dermatitis grade ≥2 with moist desquamation or intense pain within the control breast-compartment were prescribed topical corticosteroids (Advantan lotion, 0.1% Methylprednisolone aceponate, Jenapharm GmbH & Co. KG, Jena, Germany), if necessary.
Radiation protocol
Patients received a fractionation regimen of 50 Gy in 25 fractions (patients younger than 65 years with pathology-confirmed invasive breast cancer received a sequential boost up to 66 Gy with 2 Gy/fx) using 6 MV or a combination of 6 MV and 10 MV photons following 3D planning, either with tangential photon beams as sliding-window intensity modulated radiotherapy (IMRT) or volumetric arc therapy (VMAT). All patients were treated with a Varian TrueBeam STx (Palo Alto, CA) linear accelerator in a supine position and with their arms above their head. International Commission on Radiation Units and Measurements (ICRU) recommendations with dose limits of 95–107% were followed.
Patient evaluation
Patients were evaluated at weekly intervals during therapy; radiation dermatitis assessments were reported corresponding to the RTOG/EORTC toxicity criteria [Citation21]. Radiation dermatitis RTOG/EORTC scores are classified as follows; Grade 1: faint erythema or dry desquamation, Grade 2: moderate to brisk erythema; patchy moist desquamation, mostly confined to skin folds and creases; Grade 3: moist desquamation other than in skin folds and creases; Grade 4: skin necrosis or ulceration of full thickness dermis.
Upon completion of radiation treatment (50 Gy/25 fx), Hydrofilm dressings were removed and the patient-related modified Radiation-Induced Skin Reaction Assessment Scale (RISRAS) was recorded by the patient itself [Citation11,Citation14,Citation22]. For RISRAS scoring, patients assessed their maximum experience of itchiness, burning, pain and limitations in daily activities. For each of those items 0–3 points were given by the patient (0 = not at all, 1 = a little, 2 = quite a bit, 3 = very much).
The day after completion of radiation treatment, digital photographs (Canon EOS 1300 D, Canon Inc., Tokyo, Japan) were taken, and erythema readings – five in different positions across the Hydrofilm-covered as well as the control area – were carried out with a reflectance spectrophotometer (CR-20, Konica Minolta, Marunouchi, Japan), a well-validated erythema measurement tool [Citation8,Citation23,Citation24]. This compact instrument is held directly above the patient’s skin without touching it, and measurements are performed automatically. The measurements are based on the CIE (Commission Internationale de l’Eclairage) system of tristimulus values with each color being described by three coordinates using the L*a*b* coordinate system; in this system the L* value describes the light intensity, the a* value describes the position of the color on a scale ranging from red to green and the b* value the position on a scale ranging from blue to yellow. In this system, an increase in a* values is interpreted as an increased erythema intensity [Citation25]. Momm and Russell performed the first feasibility studies on spectrophotometric skin measurements in radiotherapy: accordingly, a* values between 0 and 10 describe unirradiated skin, values of 10–15 mild erythema and values up to 25 very intense erythema [Citation23,Citation24,Citation26]. Such measurements allow for greater reliability and reproducibility than subjective human observations [Citation27,Citation28].
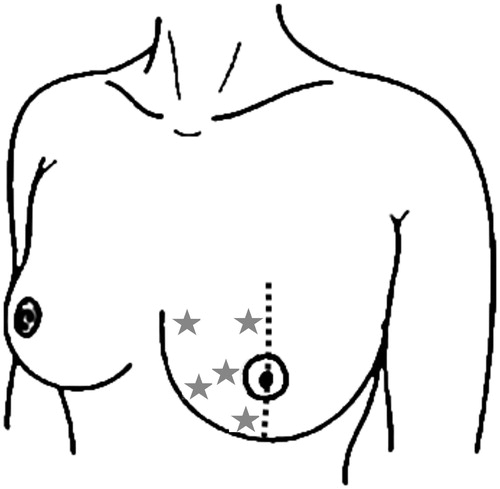
Locations of erythema measurements are shown in .
Figure 1. Locations of erythema measurements within a breast half. Measurement locations are indicated as stars. Measurements were performed analogously within the other breast half.
In those patients which required topical steroids (n = 6), spectrophotometric skin measurements were brought forward to avoid inconsistencies due to the anti-inflammatory topical therapy.
RTOG/EORTC radiation dermatitis and modified RISRAS scoring as well as the spectrophotometric erythema readings were performed solely following the application of 50 Gy in 25 fractions. Possibly increased skin reactions after local boost radiotherapy of the former tumor bed (performed in 19 patients) were not assessed for comparability reasons.
Statistical analysis
SPSS Statistics v25 (IBM, Armonk, NY) was used for data analysis. Dermatitis grading, erythema measurements and patient-assessed RISRAS scores were analyzed with the two-tailed Wilcoxon signed rank test in order to compare not normally distributed, related samples. p values <.05 were considered statistically significant.
Phantom studies
To determine possible dose variations caused by the application of Hydrofilm during radiotherapy, Percentage Depth Dose (PDD) measurements were performed with a RW3 Slab phantom (PTW, Physikalisch-Technische Werkstätten Dr. Pychlau GmbH, Freiburg, Germany) at depths of 7, 9, 11, 13, 15, 17, 20, 25, 30 and 50 mm. PDDs were measured with an Advanced Markus ion chamber (PTW), either with or without Hydrofilm on the surface with a source-to-surface distance of 100 cm. Three measurements were taken in each depth with the Unidos webline (PTW) electrometer for both 6× and 10× photon beams.
Results
Sixty-two patients were enrolled between 28 September 2016 and 22 September 2017. Fifty-six patients completed the study as initially intended and yielded data for analysis. Patient demographics are given in . Six stopped applying Hydrofilm within the first five days due to itching sensations (n = 6), redness of the skin (n = 5) and allergic eczema (n = 3), including coincidence.
Table 1. Patient baseline characteristics.
Phantom studies
Phantom studies revealed insignificantly increased doses of less than 0.1% with a clinically negligible dose build-up.
As there was no relevant effect on dose, Hydrofilm was left on the skin surface during the entire treatment period of radiation therapy.
Effect on severity of radiation dermatitis and moist desquamation as assessed by RTOG/EORTC scores
The maximum severity of radiation dermatitis was lower within the Hydrofilm-covered breast compartments when compared to the control compartments treated prophylactically with moisturizing 5% urea lotion.
Analysis of RTOG/EORTC scores showed a significantly reduced severity of radiation dermatitis in the Hydrofilm-covered breast halves compared to control breast halves. Mean and median scores were 0.35 and 0 under Hydrofilm, and 1.33 and 1 in the control compartments, respectively. Mean and median differences were 0.98 and 1.0, respectively (p ≤ .001).
Six patients developed moist desquamation in the control areas, while none was seen under the film.
We determined a reduced dermatitis severity within the Hydrofilm compartments in 50 of 56 patients, resulting in an overall response rate of 89.3%.
No significant differences in radiation dermatitis severity were found in an inter- and intra-patient comparison of dermatitis scores between the medial and lateral breast halves.
Detailed results are given in and exemplary photographs of four different patients on completion of the radiation therapy are shown in .
Figure 2. Exemplary photographs, taken from two different angles, of four (A–D) different patients following completion of the radiation therapy. In these patients, Hydrofilm was applied to the lateral breast compartment during the entire radiation therapy period.
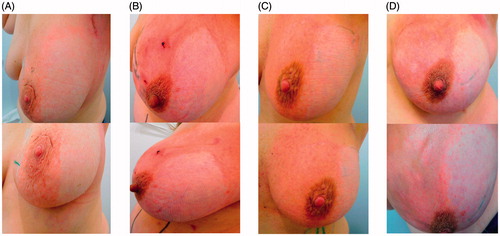
Table 2. Maximum severity of radiation dermatitis corresponding to the RTOG/EORTC score in 56 patients.
Objective evaluation of maximum erythema severity using reflectance spectrophotometry
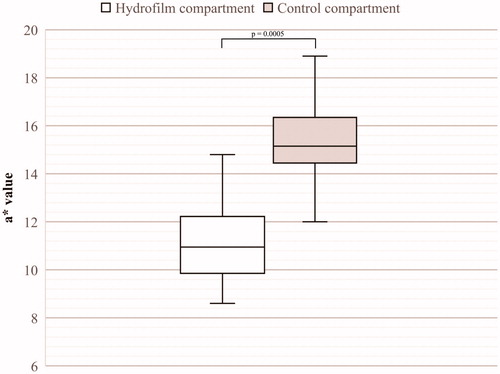
Maximum severity of erythema was significantly lower in the Hydrofilm-covered breast-halves compared with the control breast-halves: mean and median a* values were 11 and 11.1 under Hydrofilm and mean and median a* values were 16.5 and 15.3 in the control compartments, respectively (p = .0005). Differences in erythema severity are shown in .
Subjective experience of itching, burning, pain and limitations in daily activities
Patient-assessed modified RISRAS scoring showed a significantly decreased subjective experience of treatment-related itching and pain in the Hydrofilm-covered breast halves. Mean itching and pain scores were 0.32 and 0.44, compared to 1.0 and 0.83 in the control compartments. Corresponding p values were <.001 for itching and .04 for pain. No significant differences were found for burning sensations of the skin and limitations of daily activities during the treatment period with mean scores of 0.44 and 0.24 under Hydrofilm, and scores of 0.8 and 0.48 in the control compartments (respective p values were .08 and .16).
Side effects induced by Hydrofilm dressings
Adverse reactions induced by Hydrofilm were minor and can be placed into two distinct categories: side effects in the covered area under the film, and those at the edge of the film dressing resulting from local shear stress.
Three patients developed an allergic immediate reaction with local maculopapular rash, and two patients developed a localized delayed rash or allergic contact dermatitis following 1–2 weeks after the first Hydrofilm application.
Mild skin redness, burning sensations and itching in the Hydrofilm area were seen in 12, 7 and 10 patients, but in each case exclusively present at the very edge of the film dressing. In all cases, this was due to shear stress from inaccurate and/or non-smooth application of Hydrofilm dressings. Unfortunately, in these cases shear stress was not immediately apparent to the radiation therapist, so in three of these patients we observed small blisters (<1 cm diameter) and in one patient a large blister (>1 cm diameter) – all at the peripheral zone of Hydrofilm. Frequencies of observed adverse reactions are shown in .
Table 3. Side effects caused by Hydrofilm dressings in 62 patients.
Cost–benefit consideration
On average, costs from Hydrofilm dressings (mean 3 dressings per patient) were approximately 10 €. In contrast, costs for prophylactical treatment of the control breast-halves with Eucerin UreaRepair PLUS Lotion 5% were approximately 20 €.
Patients required more frequent patient visits and radiation therapist time because of increased skin injury in the non-film covered halves. In six cases, additional costs arose from topical corticosteroids (approximately 25 €per patient) and one of these patients also required inpatient treatment due to extensive moist desquamation.
Discussion
The female breast is especially susceptible to skin damage during radiotherapy because of the mechanical/physical irritation from proximity to clothing, frequent movement of the arms, and perspiration. While protection from friction and maceration is standard of care in slow healing wounds, this has not been widely appreciated in radiation-induced skin injury [Citation29–31].
The application of a protective layer on the skin surface may reduce such friction by deflecting shear forces to a larger skin surface, thereby reducing damage to the subjacent basal-stem layer, which, because of its rapidly dividing skin stem-cells, is predestined for radiotherapy-induced injury. Such a cover-protected skin regeneration environment was also hypothesized to maintain a scaffold for intradermal cell migration and accelerated reepithelialization [Citation12–14]. The maintained skin hydration underneath a semi-permeable dressing like Hydrofilm may additionally improve skin regeneration [Citation13].
In the management of radiation-induced skin injury, inconsistent data have been obtained in studies carried out with gentian violet dressings, hydrogel dressings and silver nylon dressings [Citation32–34]. Three recent studies assessed the efficacy of foam dressings (Mepilex lite) and showed a decreased severity in overall skin reactions by 40%, and reduced erythema severity by 30% in irradiated breast cancer patients [Citation11,Citation30,Citation35]. Decreased healing times in moist desquamation from 23 to 16 days were observed in irradiated head and neck cancer (HNSSC) patients [Citation16].
So far, there are merely four studies investigating the effect of dressings in terms of a preventive application during photon radiation therapy. In two studies with patients undergoing postmastectomy irradiation, Cavilon spray-on no sting barrier film was evaluated, a polymeric solution forming a uniform film when applied to the skin and which usually is intended as a primary barrier against irritation from body fluids. An initial study found the frequency of moist desquamation reduced from 46% to 33% and reduced dermatitis grades, but a subsequent and larger scaled study using a slightly modified cream formulation reported neither a statistical difference of skin reactions nor moist desquamation rates between treatment and control arms [Citation12,Citation13]. In two more recent and promising studies, Herst et al. evaluated prophylactically applied silicone-dressings compared with aqueous cream during adjuvant radiotherapy of HNSSC and breast cancer patients; in HNSSC patients, skin reaction severity was decreased by 29% and moist desquamation rates by 37% in a Chinese cohort and by 27% and 28%, respectively, in a Caucasian cohort [Citation36]. Of particular interest are the results obtained in breast cancer patients demonstrating a significant decrease in peak skin reactions: within the control areas patients developed grade 0, I, II and III skin reactions in 0%, 28%, 64% and 8%, respectively, whereas 56%, 36% and 8% of patients developed grade 0, I and III reactions following prophylactic treatment with silicone-patches. Decreased moist desquamation rates from 26% to 0% for were also reported [Citation14].
This study is the first evaluating the efficacy of prophylactically applied (Hydrofilm) polyurethane dressings and extends those previously performed trials by substantiating the physician-assessed observations with reflectance spectrophotometry allowing for objective and precise measurements [Citation8,Citation23,Citation24,Citation26].
Our results are in accordance with recent research data on barrier-forming products in radiotherapy, and thus corroborate the assumed principle of prophylactic superficial skin protection for the reduction or even prevention of radiation dermatitis. Maximum dermatitis severity in our control skin compartments was comparable to the recent literature and moist desquamation rates were slightly lower (10.7%) [Citation8,Citation13,Citation14,Citation19]. In the Hydrofilm-covered integument, we found a significantly reduced severity of radiation dermatitis with a reduction in maximum RTOG/EORTC scores by 1.0 and significantly reduced erythema intensities with an overall response rate of approximately 90%. Even more important: the patients’ subjective experience of itching and pain was also significantly lower in those skin areas covered with Hydrofilm.
It was further determined that due to its hardly detectable bolus effect and high wearing comfort, Hydrofilm could remain on the skin surface during the entire therapy period without any impairments of the radiation treatment and daily activities such as showering, workout or wardrobe selection.
Because of its favorable cost–benefit ratio, a preventive application of Hydrofilm dressings also appears economically attractive. Average costs were lower than for our standard skin care and subsequent costs for further treatments like topical steroids were saved.
Side reactions were minor and, in all cases, self-limiting without additional therapy; most of these were caused by shear stress at the edges of sub-optimally attached film dressings. Accordingly, these adverse reactions appeared within 24 h following the attachment of Hydrofilm dressings, and required appropriate film dressing replacement. Due to the patient’s individual anatomy, for example, because of varying skin folds, a smooth and shear stress-free application was not always possible and mostly there was no obvious shear stress discernible to the radiation therapist in these cases. The strata of skin around the stratum spinosum are most susceptible to shear, particularly in already damaged skin. When the stratum spinosum separates from the connecting tissues below, plasma diffuses out and eventually forms blisters [Citation37]. Such shear-stress induced blisters represent the most serious side effect observed; they were seen in four women accounting for approximately 7% (4/56) of the patient cohort. Except for localized allergic or not-specified intolerance reactions, which were present in 8.1% (5/62) of patients, adverse reactions might have been avoided by an appropriate Hydrofilm application, especially in the final phase of radiation therapy when radiation-induced skin damage is already advanced and the skin is, therefore, more susceptible to shear. However, in a clinical routine setting, the use of larger-scaled dressings could possibly shift the shear-forces at the film dressing edges to fully intact skin areas next to the treatment area and thereby avoid or at least reduce the observed reactions.
Advantages of this study are the highly uniform treatment site and no varying fractionation schedules as measurements were exclusively performed following 50 Gy/25 fx. The intra-patient randomization design excludes other variables (like patient-related aspects) possibly influencing our findings. And particularly the use of validated scoring and objective technical measurement instruments render the data quite robust. However, because of the visibility of Hydrofilm on the skin-surface, a blinding of both patients and health care providers was not feasible, which might have biased the physician-assessed dermatitis scorings. Another limitation is that no measurements were performed at the expected dermatitis peak 1–2 weeks after therapy completion in order to ensure reliability of the obtained data because 19/62 patients received a local boost radiotherapy of up to 66 Gy. In conclusion, our study demonstrates that Hydrofilm significantly reduced radiation induced skin injury when applied prophylactically. We observed a significant beneficial effect on the maximum severity of radiation dermatitis, erythema as well as subjective itching and pain experience. Adverse reactions were only minor. In view of a favorable cost–benefit ratio and the (mostly) easy and quick application, a prophylactic application of Hydrofilm polyurethane dressings in adjuvant radiotherapy of breast cancer patients seems to be justified.
Acknowledgments
The authors wish to thank the Paul Hartmann AGfor donating Hydrofilm dressings and the Rembrandtin Powder Coating GmbH, Germany, for generously providing the photospectrometer free of charge.
Disclosure statement
The authors declare that they have no conflicts of interest.
References
- van Dam PA, Tomatis M, Marotti L, et al. Time trends (2006–2015) of quality indicators in EUSOMA-certified breast centres. Eur J Cancer. 2017;85:15–22.
- Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085–2092.
- Bray FN, Simmons BJ, Wolfson AH, et al. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther. 2016;6:185–206.
- Chan RJ, Webster J, Chung B, et al. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2014;14:53.
- Lin LC, Que J, Lin LK, et al. Zinc supplementation to improve mucositis and dermatitis in patients after radiotherapy for head-and-neck cancers: a double-blind, randomized study. Int J Radiat Oncol Biol Phys. 2006;65:745–750.
- Maiche A, Isokangas OP, Grohn P. Skin protection by sucralfate cream during electron beam therapy. Acta Oncol. 1994;33:201–203.
- Wells M, Macmillan M, Raab G, et al. Does aqueous or sucralfate cream affect the severity of erythematous radiation skin reactions? A randomised controlled trial. Radiother Oncol. 2004;73:153–162.
- Hindley A, Zain Z, Wood L, et al. Mometasone furoate cream reduces acute radiation dermatitis in patients receiving breast radiation therapy: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2014;90:748–755.
- Rollmann DC, Novotny PJ, Petersen IA, et al. Double-blind, placebo-controlled pilot study of processed ultra emu oil versus placebo in the prevention of radiation dermatitis. Int J Radiat Oncol Biol Phys. 2015;92:650–658.
- Sharp L, Finnila K, Johansson H, et al. No differences between Calendula cream and aqueous cream in the prevention of acute radiation skin reactions – results from a randomised blinded trial. Eur J Oncol Nurs. 2013;17:429–435.
- Diggelmann KV, Zytkovicz AE, Tuaine JM, et al. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol. 2010;83:971–978.
- Graham P, Browne L, Capp A, et al. Randomized, paired comparison of no-sting barrier film versus sorbolene cream (10% glycerine) skin care during postmastectomy irradiation. Int J Radiat Oncol Biol Phys. 2004;58:241–246.
- Graham PH, Plant N, Graham JL, et al. A paired, double-blind, randomized comparison of a moisturizing durable barrier cream to 10% glycerine cream in the prophylactic management of postmastectomy irradiation skin care: trans Tasman Radiation Oncology Group (TROG) 04.01. Int J Radiat Oncol Biol Phys. 2013;86:45–50.
- Herst PM, Bennett NC, Sutherland AE, et al. Prophylactic use of mepitel film prevents radiation-induced moist desquamation in an intra-patient randomised controlled clinical trial of 78 breast cancer patients. Radiother Oncol. 2014;110:137–143.
- Thilmann C, Adamietz IA, Ramm U, et al. [Radiation load on the skin using a silicone-coated polyamide wound dressing during photon and electron radiotherapy]. Strahlenther Onkol 1996;172:270–274.
- Zhong WH, Tang QF, Hu LY, et al. Mepilex Lite dressings for managing acute radiation dermatitis in nasopharyngeal carcinoma patients: a systematic controlled clinical trial. Med Oncol. 2013;30:761.
- Palfreyman S, Stevens J. Use of hydrofilm and hydrofilm plus in the community: an assessment. Br J Community Nurs. 2010;15:S34, S36–S40.
- Weber TM, Kausch M, Rippke F, et al. Treatment of xerosis with a topical formulation containing glyceryl glucoside, natural moisturizing factors, and ceramide. J Clin Aesthet Dermatol. 2012;5:29–39.
- Momm F, Weissenberger C, Bartelt S, et al. Moist skin care can diminish acute radiation-induced skin toxicity. Strahlenther Onkol. 2003;179:708–712.
- Pardo Masferrer J, Murcia MM, Vidal FM, et al. Prophylaxis with a cream containing urea reduces the incidence and severity of radio-induced dermatitis. Clin Transl Oncol. 2010;12:43–48.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341–1346.
- Noble-Adams R. Radiation-induced skin reactions. 3: Evaluating the RISRAS. Br J Nurs. 1999;8:1305–1312.
- Momm F, Bartelt S, Haigis K, et al. Spectrophotometric skin measurements correlate with EORTC/RTOG-common toxicity criteria. Strahlenther Onkol. 2005;181:392–395.
- Russell NS, Knaken H, Bruinvis IA, et al. Quantification of patient to patient variation of skin erythema developing as a response to radiotherapy. Radiother Oncol. 1994;30:213–221.
- Kuehni RG. Color-tolerance data and the tentative CIE 1976 L a b formula. J Opt Soc Am. 1976;66:497–500.
- Wengstrom Y, Forsberg C, Naslund I, et al. Quantitative assessment of skin erythema due to radiotherapy – evaluation of different measurements. Radiother Oncol. 2004;72:191–197.
- Westerhof W, van Hasselt BA, Kammeijer A. Quantification of UV-induced erythema with a portable computer controlled chromameter. Photodermatol. 1986;3:310–314.
- Tesselaar E, Flejmer AM, Farnebo S, et al. Changes in skin microcirculation during radiation therapy for breast cancer. Acta Oncol. 2017;56:1072–1080.
- Arroyo AA, Casanova PL, Soriano JV, et al. Open-label clinical trial comparing the clinical and economic effectiveness of using a polyurethane film surgical dressing with gauze surgical dressings in the care of post-operative surgical wounds. Int Wound J. 2015;12:285–292.
- Herst PM. Protecting the radiation-damaged skin from friction: a mini review. J Med Radiat Sci. 2014;61:119–125.
- Pastore F, Conson M, D'Avino V, et al. Dose-surface analysis for prediction of severe acute radio-induced skin toxicity in breast cancer patients. Acta Oncol. 2016;55:466–473.
- Aquino-Parsons C, Lomas S, Smith K, et al. Phase III study of silver leaf nylon dressing vs standard care for reduction of inframammary moist desquamation in patients undergoing adjuvant whole breast radiation therapy. J Med Imag Radiat Sci. 2010;41:215–221.
- Gollins S, Gaffney C, Slade S, et al. RCT on gentian violet versus a hydrogel dressing for radiotherapy-induced moist skin desquamation. J Wound Care. 2008;17:268–270. 272, 274–275.
- Niazi TM, Vuong T, Azoulay L, et al. Silver clear nylon dressing is effective in preventing radiation-induced dermatitis in patients with lower gastrointestinal cancer: results from a phase III study. Int J Radiat Oncol Biol Phys. 2012;84:e305–e310.
- Fernandez-Castro M, Martin-Gil B, Pena-Garcia I, et al. Effectiveness of semi-permeable dressings to treat radiation-induced skin reactions. A systematic review. Eur J Cancer Care. 2017;26. DOI:10.1111/ecc.12685
- Wooding H, Yan J, Yuan L, et al. The effect of Mepitel Film on acute radiation-induced skin reactions in head and neck cancer patients: a feasibility study. Br J Radiol. 2018;91:20170298.
- Sulzberger MB, Cortese TA, Fishman L, et al. Studies on blisters produced by friction. I. Results of linear rubbing and twisting technics. J Invest Dermatol. 1966;47:456–465.