Abstract
Introduction: Adjuvant endocrine therapy improves recurrence-free and overall survival in primary breast cancer. However, not all patients complete their planned treatment, mostly because of side-effects. The aim of this study was to examine the adherence to adjuvant endocrine therapy in a cohort of primary breast cancer patients in Region Jönköping County, Sweden, after 3 and 5 years.
Material and methods: The Swedish Breast Cancer Register was used to identify patients diagnosed with hormone receptor positive breast cancer in Region Jönköping County between 2009 and 2012. Adherence was evaluated based on data from the Swedish Prescribed Drug Register, and Medication Possession Ratio (MPR), defined as the days’ supply of medication during the period from the first dispensing till the last dispensing in the time period (3 and 5 years), divided by number of days. Adherence was defined as MPR ≥80%. Regression analyses were used to identify subgroups associated with adherence; age, type of endocrine treatment, additional adjuvant therapy, and hospital responsible for the follow-up (Eksjö, Jönköping, and Värnamo).
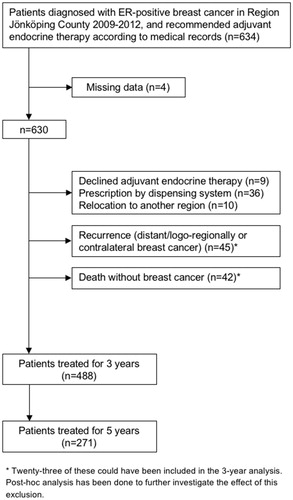
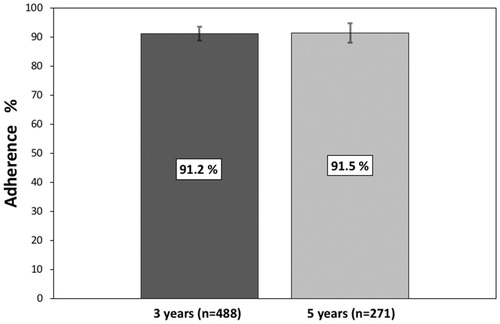
Results: We identified 634 patients who were recommended adjuvant endocrine therapy and to be able to estimate adherence after 3 and 5 years, 488 patients were included in the analysis. After 3 years of treatment, 91.2% of the patients (95% confidence interval (CI) 88.7–93.6; n = 445), were found to be adherent. The corresponding figure for the 271 patients who had completed 5 years of treatment was 91.5% (95% CI 88.2–94.8; n = 248). No subgroups (age, endocrine therapy, radio/chemotherapy, or hospital) were significantly associated with adherence in the multiple logistic regression analysis.
Discussion: This study shows substantially higher adherence to adjuvant endocrine therapy than previously reported. Reasons for this could be differences in routines for therapy information and follow-up, but this needs to be further investigated.
Introduction
Approximately 85% of all primary breast cancers express estrogen receptors (ER) and these patients are generally recommended adjuvant endocrine therapy, such as tamoxifen or an aromatase inhibitor (AI) [Citation1,Citation2]. Tamoxifen inhibits ER-positive breast cancer by competitively binding to ER and is effective irrespective of menopausal status. AIs inhibit the aromatase enzyme thereby reducing estrogen levels in the blood. To be effective in premenopausal women, AIs have to be combined with ovarian suppression to inhibit the positive feedback system regulated by the hypothalamus and the pituitary gland [Citation3,Citation4].
Five years of tamoxifen reduces the recurrence rate by 39% at 15 years in patients with ER-positive breast cancer, compared with no endocrine therapy, and the corresponding figure regarding breast cancer mortality is 30% [Citation5]. AI is significantly more effective compared with tamoxifen in postmenopausal women [Citation6]. Nowadays, extended treatment is often recommended to patients with a higher risk of recurrence [Citation7–10]. A recent meta-analysis has shown a risk of recurrence and breast cancer mortality up to 20 years, in patients treated with 5 years of adjuvant tamoxifen therapy, strongly correlated to tumor size and nodal status [Citation11].
Despite the positive effects of endocrine treatment, many patients stop taking their prescribed medicine, mostly because of side-effects. Tamoxifen and AIs have side effects similar to those often seen in menopause, such as hot flashes and vaginal dryness. Musculoskeletal symptoms may be associated with both drugs, but are more frequently reported during treatment with AIs [Citation12]. Moreover, AIs increase the risk of osteoporosis and bone fractures and the risk of these events increases with longer treatment [Citation6]. Tamoxifen is associated with an increased incidence of venous thromboembolism as well as proliferative endometrial abnormalities with increased risk of endometrial cancer [Citation13,Citation14].
Previous studies investigating the adherence to endocrine therapy in breast cancer differ in their methods and definitions. They may be based on pharmacy databases, medical record reviews, patient self-reports, interviews, prospective trials, or assessments of drug concentrations. Studies based on register/databases report a wide range of adherence [Citation15–17].
Region Jönköping County has a population of approximately 340,000 and about 300 patients are diagnosed with primary breast cancer each year, including DCIS (ductal carcinoma in situ). As a result of centralization of breast cancer care in the region, the Department of Oncology, County Hospital Ryhov, Jönköping, has been responsible for the follow-up of all patients with invasive breast cancer since 2016. However, before that the responsibility for follow-up was shared between all three hospitals in the region; the Department of Oncology, County Hospital Ryhov, Jönköping, the Department of Surgery, Highland Hospital, Eksjö, and the Department of Surgery, Värnamo Hospital, Värnamo.
The aim of this study was to examine the adherence to adjuvant endocrine treatment in Region Jönköping County, Sweden, after 3 and 5 years, in patients diagnosed with primary breast cancer between 2009 and 2012. A secondary aim was to investigate the adherence in different subgroups.
Material and methods
Definition of adherence
Different terms are used for specifying how well patients follow prescription recommendations. Adherence, compliance, and persistence are terms often used in studies for this purpose. The word adherence will be used in this article, indicating the extent to which patients follow the recommendation of endocrine treatment for a specific time period. MPR (Medication Possession Ratio) is a common definition in adherence analyses and implies the ratio of total days´ supply to the number of days in the observation period. The lower limit of ≥80% is often used to define if the patient is adherent [Citation18]. We, therefore, chose to define adherence as coverage by available medication of at least 80% of the days during the 3-year and 5-year periods.
Patients and methods
The Swedish Breast Cancer Register contains information of patient and tumor characteristics, adjuvant therapy, and follow-up data [Citation19]. Patients who were diagnosed with ER-positive breast cancer in Region Jönköping County between 1 January 2009 and 31 December 2012, were identified using the Swedish Breast Cancer Register (n = 802). Review of medical records was performed to identify patients that were recommended adjuvant therapy (n = 634). According to the former Swedish guidelines, patients were generally recommended adjuvant therapy if they had an ER-positive tumor >10 mm and/or lymph node positivity. Forty-five patients were excluded because they either declined adjuvant endocrine treatment (n = 9) or were prescribed the treatment by a dispensing system (n = 36). As our aim was to investigate adherence in our region after the specific time points of 3 and 5 years, and not adherence up to any event that would imply end of treatment or lost to follow-up, patients were also excluded for the following reasons; relocation to another health care region (n = 10), death without breast cancer during follow-up (n = 42), and recurrence or contralateral breast cancer during the planned treatment period (n = 45), as this was expected to alter treatment recommendations. However, as a post-hoc analysis, medical records for the excluded patients were reviewed to estimate the impact of the exclusion. Another four patients were accidentally not included in the adherent analyses for the following reasons; dispersing system initiated after 5 years of treatment, ER-negative contralateral relapse, relocation to another region although Region Jönköping County still prescribed treatment, and one was missed in the prescription data request. In total, 488 patients were included in the analyses ().
The routines for follow-up differed slightly among the different hospitals. Generally, at the start of treatment the patients were carefully informed about the endocrine therapy effects and side effects. Written information was handed out, and the patients were encouraged to contact the clinical department for discussion of treatment issues. After 1 year, there was a follow-up visit to a doctor, and a yearly mammography was performed. At the Department of Surgery, Eksjö, a nurse contacted the patient after 3 months and yearly to give the mammography results and at the same time follow up on the endocrine treatment and make sure a new prescription was available. At the Department of Surgery, Värnamo, the doctor yearly wrote a letter to inform the patient about the mammography results and that a new prescription of the endocrine drug had been prescribed. At the Department of Oncology, Jönköping, a nurse initially called patients once a year for support, but this follow up was ended in 2013. According to our new local guidelines, patients are encouraged to call the clinic for support and if they have any questions as well as for new prescriptions, and they are asked not to stop therapy without discussing it with their oncology nurse or treating physician.
The Swedish prescribed drug register
The Swedish Prescribed Drug Register has been in use since 1 July 2005 and contains data about a patient’s prescribed drugs, such as costs, dates of prescription and dispensing, Anatomical Therapeutic Chemical Classification (ATC)-codes, active substances, number of packages and Defined Daily Doses (DDD) [Citation20]. Drugs are coded according to the ATC-code. DDD is the average daily dose when the drug is used according to its main indication. It is a technical measurement used in statistical analyses and is often used in combination with the ATC-system. For the present study, data regarding the following information were retrieved; dates of prescription and dispensing, number of packages, ATC-code, product name, substance name, DDD for each prescription, and DDD for each package (28 October 2016). We chose to focus on the adherence of oral endocrine treatment, and therefore, omitted to include information regarding GnRH-analogues.
Ethics
This study was approved by the Regional Ethical Review Board in Linköping, registration number 2016/61-31 (approved 24 February 2016).
Statistical analysis
Our primary endpoint was adherence after 3 and 5 years. We used the date of the first prescription to determine the treatment periods of 3 and 5 years. All collected medications from the first dispensing till the last one before the end date in the respective time periods were added and the total DDD for all endocrine drugs were summarized. The sum was divided by the days in the time period (3 × 365 or 5 × 365 d). Patients were defined as adherent if this ratio was ≥80% for the 3-year and 5-year periods. As data cutoff from the drug register was 28 October 2016, patients who collected their first prescription after 28 October 2011 were not included in the 5-year analysis. Bootstrapping (resampling 1000 times) was used to calculate confidence interval (CI) for adherence of the 3-year and 5-year analyses.
To estimate the degree of non-adherence, the MPR of the non-adherent population was subdivided into three different subgroups; 0–30%, 31–60%, and 61–79% and the percentage distribution was calculated.
The following subgroups were investigated; age at time for diagnosis (<40, 40–59, 60–79, ≥80), adjuvant radiotherapy (yes/no), adjuvant chemotherapy (yes/no), hospital responsible for the follow-up; (Eksjö/Jönköping/Värnamo), and treatment category (AI/tamoxifen/sequenced tamoxifen and AI). In patients who had been treated for <5 years, the type of endocrine prescribed during the first 3 years was recorded in the sub-group analysis. If a patient had switched endocrine treatment at any point during the period, the patient was recorded as having sequenced therapy. To calculate predictors of adherence, binary logistic regression analyses were performed to estimate odds ratios (OR´s), corresponding confidence intervals and p-values. Both univariate and multiple logistic regression analyses were performed. Microsoft Excel 2010 and 2016 and IBM® SPSS® Statistics Version 24 (SPSS, Chicago, IL, USA) were used to handle data and perform calculations.
Results
Of the 488 patients who had been treated for 3 years, 91.2% (95% CI 88.7–93.6; n = 445) were defined as adherent. Of the 271 patients who had reached a treatment period of 5 years, 91.5% (95% CI 88.2–94.8; n = 248) were defined as adherent for the full treatment period ().
Figure 2. Percentage of patients adherent to endocrine treatment after 3 years (91.2%; 95% CI 88.7–93.6) and 5 years (91.5%; 95% CI 88.2–94.8), respectively.
Radiotherapy was significantly associated with adherence in the 3-year analysis according to univariate regression (OR 1.9, 95% CI (1.0–3.6), p = .041). However, multiple logistic regression analyses did not find any significant association (p values ≥.05) with age at time of diagnosis, the type of endocrine treatment, if radio/chemotherapy was/were given or to the hospital responsible for the follow-up (). The analysis of the degree of non-adherence is presented as descriptive data ().
Table 1(A). Predictors of adherence to adjuvant endocrine treatment after 3 years (n = 488) according to univariate and multiple logistic regression.
Table 1(B). Predictors of adherence to adjuvant endocrine treatment after 5 years (n = 271) according to univariate and multiple logistic regression.
Table 2. Descriptive data of medication possession ratio (MPR) among non-adherent patients after 3 (n = 43) and 5 years (n = 23), respectively.
Discussion
Endocrine adjuvant therapy is known to be effective in preventing relapse and reducing mortality in primary ER-positive breast cancer [Citation5,Citation6]. Decreased levels of adherence are associated with increased risk of death and it is therefore of utmost importance to ensure that patients actually take their prescribed therapy [Citation21,Citation22]. In the present study, adherence exceeded 90% after both 3 and 5 years and we hereby show that very good adherence to adjuvant endocrine therapy in primary breast cancer can be achieved. The degree of non-adherence, according to descriptive data of the three different MPR groups, was similar among the patients after 3 years. However, after 5 years, as expected, there was a trend towards more non-adherent patients with lower MPR values.
Most previous studies have reported significantly lower figures. Kimmick and colleagues reported adherence as low as 60% after 1 year in low-income patients with primary breast cancer [Citation23]. Another study found an adherence range of 50–68% after 3 years [Citation24]. Retrospective studies based on databases and registers have shown adherence around 60–82% and 46–73% after 3 and 5 years, respectively [Citation15,Citation16]. However, as different methods and definitions are used to define adherence, it is difficult to directly compare results from different studies. Adherence might strictly be based on MPR, calculating MPR over all time periods, or annually. In this study, MPR was calculated after 3 and 5 years.
Another Swedish study by Wigertz et al., using a similar design, included 1741 patients from two other regions (Uppsala/Örebro and Stockholm/Gotland) and showed 69% adherence after 3 years [Citation17]. Despite the smaller size of our study, the confidence intervals are not overlapping. Since the follow-up routines were not presented in their study, we were not able investigate if the differences in adherence were related to the follow-up routines.
A limitation in this study is the small sample size, which does not allow identification of differences among patient groups in the subgroup analyses. However, the confidence intervals for the main results after 3 and 5 years are small and even the lower limits of 88.2% and 88.7% are well beyond earlier described results. There were trends towards higher adherence among those given radio/chemotherapy and less adherence in the youngest and oldest age groups, which is consistent with earlier studies [Citation25,Citation26]. One reasonable explanation could be that those patients receiving additional adjuvant treatment had more established contact with specialized health care, and were engaged in more discussions about their adjuvant treatment, which could positively affect adherence. Even though the follow-up routines differed somewhat between the three departments in the region, no significant difference could be seen in adherence. Another limitation is the assumption that a patient actually take the pills they have collected. The way MPR is defined and calculated is also associated with risks of error. Even if register data of prescription are a reliable source of information, there is a risk of overestimation of the true adherence when a patient may have had extra prescriptions during the measurement interval. Further, if patients switch endocrine drugs, there is a risk of a similar type of error due to overlap of prescribed tablets. In the definition of MPR in this study, the last prescription´s surplus of days´ supply exceeding the observation period, was not subtracted from the total days´ supply in the time period. This could positively affect the adherence, depending on how close the last prescription was to the final date of the observational time period.
Twenty-three patients who were excluded due to recurrence or death without breast cancer could have been included in the 3-year analysis. To investigate the potential effect of the selection bias from this exclusion, medical records were reviewed for these patients. According to medical records, which include information of prescribed drugs from all health care providers in our region, 18 patients (78%) were still prescribed endocrine therapy at time for the event of recurrence or death. If these patients had been included in the 3-year analysis, it would not have significantly affected the final results.
The strengths of this study are that it is population-based and that the calculation of adherence is based on data from the Swedish Prescribed Drug Register. Data from sources such as questionnaires or interviews seem to have low concordance to register data [Citation27], and adherence in clinical trials might be biased by factors such as monitored and motivated patients. Moreover, patient self-report and pill counts have been found to overestimate adherence [Citation28]. Thus, the results reported in this register study are less subjective. Another strength is the additional review of the medical records.
Even though one would aim at 100% adherence, it is not achievable nor preferable since endocrine treatment may severely impair quality of life for some patients. Patients who do not manage to complete full endocrine treatment due to side effects, should, however, be encouraged to complete at least 2 years of tamoxifen treatment, which has been shown to significantly improve survival after >25 years of follow-up [Citation29]. There is no simple explanation for the high adherence observed in Region Jönköping County, but, based on the follow-up routines, regular doctor visits do not appear to be necessary. Instead it seems more important to provide patients with clear information regarding the reason for treatment, possible side effects and to encourage patients not to stop treatment before discussing it with their nurse or treating physician. The importance of communication about the planned treatment has also been shown by others [Citation30]. The economic perspective may also be of importance as there seems to be an association between higher out-of-pocket costs and lower adherence [Citation31]. In Sweden, all residents receive pharmaceutical benefits meaning reduced out-of-pocket costs for prescribed medication, which may affect the level of adherence.
Our results warrant larger future studies to more carefully study subgroups to identify predictors of adherence. Increased knowledge in this area may help to focus resources on the right patient categories and to guide us to find a better structure for breast cancer treatment organization and follow-up.
Acknowledgments
Authors thank the Swedish Regional Cancer Centre South-East, for population data collection, and to administrator Ingalill Jönsson at the Department of Oncology, Ryhov County Hospital, Jönköping, Sweden, for practical help.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Cancercentrum.se [Internet]. Sweden: Cancercentrum; 2017. [cited 2017 Sep 24]. Available from: http://www.cancercentrum.se/samverkan/cancerdiagnoser/brost/kvalitetsregister/.
- Morigi C. Highlights from the 15th St Gallen international breast cancer conference 15–18 March, 2017, Vienna: tailored treatments for patients with early breast cancer. Ecancer. 2017;11:732.
- Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–1618.
- Ma CX, Reinert T, Chmielewska I, et al. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015;15:261–275.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352.
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816.
- Gray RG, Rea D, Handley K, et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. 2013;31:2013:5.
- Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–1271.
- Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375:209–219.
- Pan H, Gray R, Braybrooke J, et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–1846.
- Thürlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757.
- Braithwaite RS, Chlebowski RT, Lau J, et al. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18:937–947.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717.
- Weaver KE, Camacho F, Hwang W, et al. Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low income women. Am J Clin Oncol. 2013;36:181.
- Huiart L, Dell’Aniello S, Suissa S. Use of tamoxifen and aromatase inhibitors in a large population-based cohort of women with breast cancer. Br J Cancer. 2011;104:1558–1563.
- Wigertz A, Ahlgren J, Holmqvist M, et al. Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: a population-based study. Breast Cancer Res Treat. 2012;133:367–373.
- Raebel MA, Schmittdiel J, Karter AJ, et al. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51:S11–S21.
- Cancercentrum.se [Internet]. Sweden: Cancercentrum. 2016. [cited 2016 Oct 02]. Available from: http://www.cancercentrum.se/samverkan/cancerdiagnoser/brost/kvalitetsregister/dokument/.
- Socialstyrelsen.se [Internet]. Sweden: Socialstyrelsen; 2016. [cited 2016 Oct 02]. Available from: https://www.socialstyrelsen.se/register/halsodataregister/lakemedelsregistret.
- Makubate B, Donnan P, Dewar J, et al. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer. 2013;108:1515–1524.
- Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537.
- Kimmick G, Anderson R, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. JCO. 2009;27:3445–3451.
- Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562.
- Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. JCO. 2003;21:602–606.
- Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. JCO. 2010;28:4120–4128.
- Font R, Espinas JA, Gil-Gil M, et al. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. Br J Cancer. 2012;107:1249–1256.
- Waterhouse DM, Calzone KA, Mele C, et al. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11:1189–1197.
- Ekholm M, Bendahl P-O, Fernö M, et al. Two years of adjuvant tamoxifen provides a survival benefit compared with no systemic treatment in premenopausal patients with primary breast cancer: long-term follow-up (>25 years) of the Phase III SBII: 2pre Trial. JCO. 2016;34:2232–2238.
- Davidson B, Vogel V, Wickerham L. Oncologist-patient discussion of adjuvant hormonal therapy: results of a linguistic study focusing on patient adherence/persistence. J Support Oncol. 2007;5:139–143.
- Riley GF, Warren JL, Harlan LC, et al. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare Part D. MMRR. 2011;1:001–004.