Abstract
Background: Magnetic resonance imaging (MRI) is the most comprehensive imaging modality for radiation therapy (RT) target delineation of most soft tissue tumors including prostate cancer. We have earlier presented step by step the MRI-only based workflow for RT planning and image guidance for localized prostate cancer. In this study we present early clinical experiences of MRI-only based planning.
Material and methods: We have analyzed the technical planning workflow of the first 200 patients having received MRI-only planned radiation therapy for localized prostate cancer in Helsinki University Hospital Cancer center. Early prostate specific antigen (PSA) results were analyzed from n = 125 MRI-only patients (n = 25 RT only, n = 100 hormone treatment + RT) and were compared with the corresponding computed tomography (CT) planned patient group.
Results: Technically the MRI-only planning procedure was suitable for 92% of the patients, only 8% of the patients required supplemental CT imaging. Early PSA response in the MRI-only planned group showed similar treatment results compared with the CT planned group and with an equal toxicity level.
Conclusion: Based on this retrospective study, MRI-only planning procedure is an effective and safe way to perform RT for localized prostate cancer. It is suitable for the majority of the patients.
Introduction
Magnetic resonance imaging (MRI) has been used for more than two decades as an important tool of radiation therapy (RT) target definition [Citation1–3], as the availability of MRI has greatly improved and the treatment planning workstations have become capable of handling 3D multimodal image data. Especially as, MRI has superior properties over computed tomography (CT) in imaging of most soft tissue targets and normal tissues, it has been included in the prostate RT target definition guideline as the most accurate modality [Citation4].
Despite its superiority from the radiation oncologist’s point of view for soft tissue target delineation, MRI is not directly suitable as a RT planning reference due to some of its inherent drawbacks: lack of tissue density information needed for dose calculation and presence of geometrical errors. These issues need to be adequately handled before MRI is ready to be a primary modality for treatment planning. Additionally, the patient geometry needs to be identical to the actual treatment position and appropriate reference images for image guidance must be generated from the MR images. The system-related distortion has been reduced significantly by recent development on MRI hardware and software and the patient-induced distortion can be minimized by appropriate sequence parameters. The geometric accuracy achieved by recently installed MR platforms is acceptable for RT planning [Citation5–11].
There are a few methods for producing the tissue density information for dose calculation. The most simple ways are either to assume everything within the body contour as water equivalent or ‘average medium’ that takes the averaged effect of non-water-equivalent tissue areas into account. The next step from these uniform models is to segment larger non-water equivalent areas, like bones, as separate internal structures and assign their density with a single value [Citation12]. The more sophisticated techniques enable generation of heterogeneous presentation for body tissue electron density (ED) information from MRI data. These techniques can apply data of a single standard MR image or combining information of multiple MR sequences. The image transformation from MR image to so called substitute CT image (sCT, also known as pseudo, synthetic or virtual CT images) can be conducted by relying on atlas-based segmentation and/or MR image intensity –based conversion to EDs [Citation13–17]. Recently, two review articles have been published by Edmund and Nyholm [Citation18] and Johnstone et al. [Citation19] which summarize the substitute CT generation and workflow with additional insight in regard to this matter. The technique developed and clinically applied in our clinic generates sCT by using intensity information of a standard MR image after automatic atlas-based bone segmentation [Citation13,Citation15–17]. We have verified the entire MRI-only based RT planning process and implemented it into the routine clinical workflow for prostate cancer patients since 2012 [Citation5,Citation6,Citation15–17,Citation20–22].
In this work we have collected the early clinical experience of MRI-only planning based RT in our hospital from the first prostate cancer patients who underwent the workflow. The MRI-only workflow is only briefly presented here as the individual steps of the procedure have been presented in earlier publications [Citation5,Citation6,Citation15–17,Citation20–22]. Additionally, the statistics and analysis of unsuccessful patient cases where MRI-only based workflow was not applicable for some technical or patient-related reasons is presented. Before clinical use all the individual steps of our MRI-only based radiation therapy workflow were carefully tested to keep the process clinically equivalent to the CT based workflow. Nevertheless, we have measured early prostate specific antigen (PSA) response of this patient group compared with the corresponding CT-planned reference group to confirm that any unknown factors related to this workflow change would not affect the treatment outcome.
Material and methods
Patients
250 patients with localized prostate cancer (n = 125 + 125) were treated with definitive gold seed image guided radiation therapy to 76 Gy with 2 Gy fractions, seminal vesicles were treated to 66 Gy in 2 Gy fractions. The Gleason score of the tumors were –6–9 and the median age was 71 years (). Before imaging three cylindrical 1.2 mm x 3 mm gold seeds (CIVCO Medical Solutions, Kalona, IA, USA) were implanted into the prostate. All patients were referred to RT from the Department of Urology, Helsinki University Hospital. The MRI-only group (n = 125) consisted of consecutive patients that were planned with MRI-only procedure where no treatment planning CT was acquired. The other (reference) group ( n = 125) was planned with both MRI and CT images where MRI images were registered to CT for target delineation and CT was used as a basis of dose calculation. The MRI-only group was planned after December 2012 and the reference group was imaged and planned before December 2012. Daily kV (planar or CBCT) or MV imaging was applied for image-guided radiation therapy (IGRT). The general MRI-only based RT workflow is demonstrated in and it is briefly described in the following chapters. Both patient groups included equal number of patients with additional hormonal therapy (n = 100) or RT only (n = 25).
Figure 1. MRI-only based RT planning workflow. (a) Presents T1/T2*-weighted in-phase image used for sCT and (d) construction. (b) Illustrates T2-weighted image applied for target delineation (e). Part (c) shows the T1/T2/T2*-weighted image adopted for gold marker verification (f). Part (g) presents the final sCT used for dose calculation (h) and IGRT with CBCT localization. Part (i) shows AP substitute DRR generated from the sCT in case IGRT is based on planar localization.
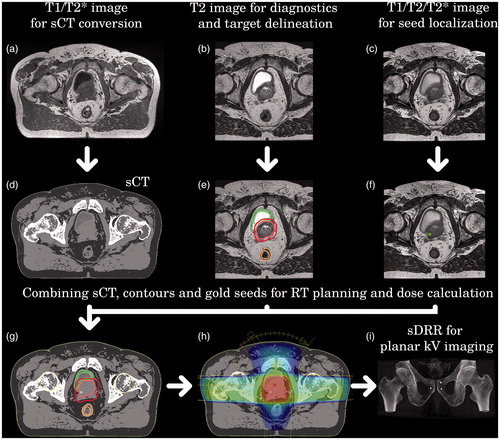
Table 1. Patient age and Gleason scores of MRI only based on planning and reference (CT) groups with RT only (monotherapy) or with additional hormonal therapy (LHRH).
Imaging
CT images were scanned with the four-slice helical CT simulator with a flat table top (GE Lightspeed RT, GE Healthcare, MARLBOROUGH, MA) using 2.5 mm slice thickness covering patient outline and pelvic structures. Patients emptied their rectum with micro-enema (5 ml MICROLAX®) before imaging and bladder size was controlled by guiding patients to empty their bladder 1 h before imaging and then drink two glasses of water.
MRI was performed using a 1.5 T wide-bore (70 cm) scanner GE Optima MR450w with the oncological package (GE Healthcare) either after similar preparations as with CT or within 30 min after CT. Imaging consisted of three sequences: 1) T2-weighted series (CUBE®, bandwidth 488 Hz/pixel) for diagnostics and target delineation, 2) T1/T2/T2*-weighted series(FIESTA C®, bandwidth 223 Hz/pixel), for seed localization and 3) T1/T2*-weighted image (LAVA Flex®, bandwidth 425 Hz/pixel) for generation of the sCT image applied for dose calculation and a reference for IGRT. Detailed description and image quality issues of these sequences have been presented in earlier studies [Citation5,Citation6]. The net precision of the MRI procedure including the effect of the flat couch top, coil frames, lasers and MRI compatible fixation was less than 2 mm within the field-of-view diameter of 42 cm.
Target delineation
We have shown earlier that when converting from a CT-based planning procedure with co-registered MR images to an MRI-only based procedure, the overall planning target volumes (PTV) are prominently reduced only in the prostate apex where CT has obvious difficulties in detection of the structure borders [Citation22]. However, the target margins and dose constraints can be retained in the MRI only workflow with the investigated sequences.
In this work the radiation oncologists had a possibility to utilize T2 MR images with both CT and MRI-only groups. No CT was available for the MRI-only group. MRI-only patients were delineated by a radiation oncologist and a urologist. In the hospital protocol, the CTV should include the prostate capsule and 2 cm of the seminal vesicles measured from the prostate base. Target delineation was performed using standard 21’’ flat screens of the Eclipse 10.0 treatment planning systems (TPS, Varian Medical Systems, CA, USA).
For the CTV1 volume of prostate and seminal vesicles a margin of 9 mm was applied in all other directions except towards rectum, where a 4 mm margin was used. For the boost volume of prostate (CTV2) a 4 mm isotropic margin was used.
Treatment planning and dose calculation of MRI-only based procedure
For the MRI-only based procedure the dose calculation was based on the sCTs generated by the dual model HU conversion technique relying on atlas-based automatic segmentation of bones followed by the HU conversion from intensity values of T1/T2* weighted in-phase MR image () [Citation15]. Within bones the conversion utilizes the observation of inversely proportional relation between MR signal and electron density and for soft tissues the electron density is generated by thresholding [Citation15,Citation16]. With current photon dose calculation algorithms (AAA/Varian Eclipse or VMC/Elekta Monaco) the calculation accuracy in the sCTs is approximately within 1% when compared to that on actual CT [Citation6,Citation15–17].
There is the lack of support of MRI-only workflow in most treatment planning software. Whenever a CT image set based ED data is required by the TPS, the sCT set labeled as ‘CT’ within the Dicom header must be created in separate software beforehand. In our case this was a semiautomatic procedure that required some extra actions between imaging and treatment planning from the user: an automatic generation of the sCT from a selected MRI set and checking the adequacy of the result.
Both patient groups were planned according to applied clinical practice and depending on the shape and size of the PTV the patients either received 330–360° VMAT with one or two arcs, seven field IMRT or three field 3DCRT with two lateral fields and one anterior arc from GA 320 to 40°. Treatment technique was not dependent on the modality of planning image set of reference whether it was MRI or CT. Criteria for target dose coverage and dose uniformity were identical in both groups.
Image guidance
RT was given with daily image guidance (IG) using gold seeds and set-up corrections were applied if larger than 3 mm deviations were noticed. For CT planned patients reference orthogonal digitally reconstructed radiographs (DRRs) were generated in a standard way so that gold seeds were visible in the images for 2D/2D IGRT. For MR planned patients substitute DRRs (sDRRs) were formed from the sCT with gold markers drawn manually as structures and masked with HU = 2000 () [Citation5,Citation21]. Kapanen et al. illustrated that gold seed localization on the T1/T2* MRI set can be performed with better than 1.5 mm accuracy in the central area of the image [Citation15]. As no geometric distortion takes place in MRI to sCT conversion related in gold seed placement, the identical accuracy is maintained. Thus, the generated reference sDRRs can be used in similar way as CT-based DRRs for image guidance. Pure T1/T2* MRI set and generated sCT are suitable for soft tissue based 3D matching with cone beam computed tomography (CBCT) based IGRT, as Korhonen et al. have shown, except bony structures that requires DRRs generated from sCT [Citation21].
MRI workflow: restrictions
Although the feasibility of MRI-only based RT planning was verified with a thorough investigation before introducing the protocol into clinical use, there were remaining restrictive issues in MRI-only workflow that could require additional CT acquisition. The concerns could be divided into system- and patient-related factors. Malfunction of MRI causing substantial changes in MR signal or artifacts could result in target delineation uncertainties or low quality sCT. Patient-related concerns were primarily major obesity in case patient would not fit into the verified field of view (FOV; 42 x 42 cm) or if having a hip implant that could introduce major metal artifacts or geometrical distortion. Accurate location of the implants should be known to avoid beams traversing through the implant. Uncertainties of gold marker identification might also require additional CT based verification. The existence of these concerns and potential additional limitations were investigated for the first 200 prostate cancer patients undergoing the MRI-only based RT workflow.
Early follow-up
As a clinical indicator for early response PSA value was determined both before (baseline value) and at the end of radiotherapy. Possible clinical acute toxicity was evaluated anamnestically and using questionnaires for genitourinary and gastrointestinal symptoms during the last doctor’s appointment at the end of the radiotherapy course.
Results
The MRI-only based workflow was successfully accomplished for 184 of the 200 (92%) first prostate cancer patients without having a planning CT acquisition. presents the reasons for the requirement of CT acquisition in the 16 of the 200 cases. In eight of these 16 cases, the reason was that one or more of the gold markers could not be identified by MRI. The reasons included the following: 1) appearance of one or more macro calcifications like the gold markers (n = 2), 2) uncertain visualization or suspicious location of a marker (n = 3) and 3) only two markers were seen in MRI (n = 3). In the latter case, however, the marker was also missing in the acquired additional CT that confirmed disappearance of the marker from the prostate. There were five patients having a hip implant causing additional CT imaging. In three of these cases the patient was turned to conventional MRI + CT workflow because the artifact of the implant overlapped the prostate and in two of the cases to accurately locate the implant for beam avoidance. Two patients were too obese for the verified FOV and thus confirmation CT was performed to verify correct body outline. In one case the MR image suffered from a motion artifact. It would have been possible to perform re-MRI but CT was acquired due to departments MRI and CT resources during the day. There were no cases, in which MR image quality in general or signal level would have caused need for additional CT acquisition.
Table 2. Reasons for requirement of additional CT acquisition for patients undergoing the MRI-only RT planning workflow.
There were no unexceptional changes in early PSA response when CT was omitted from the planning process and replaced by MRI: Monotherapy MRI group baseline median PSA 8.8 ng/ml, at the end of the treatment 5.2 ng/ml; CT group baseline 8.5 ng/ml, at the end of the treatment 5.9 ng/ml, respectively. Hormone + MRI only RT baseline median PSA was 1.2 mg/ml, at the end of the treatment it was 0.2 ng/ml. Hormone + CT based RT baseline median PSA was 1.3 ng/ml, at the end of the treatment it was 0.3 ng/ml. All acute genitourinary and gastrointestinal toxicity during radiotherapy was mild in the MRI-only and CT + MRI patient groups. There was no RTOG grade 3-4 toxicity in any of the patients. This result is expected as no changes in target volume definition or the treatment technique were performed. Longer follow-up is needed, however, to assess the oncological outcome of the patients in our study.
Discussion
Our clinical data showed that MRI-only based RT planning workflow can be successfully accomplished for clear majority of the prostate cancer patients. The MRI-only workflow was limited mostly by uncertain identification of the gold markers. Additional limitations were large image artifacts caused by hip implants and patient obesity. No unexpected limitations were found. The first limitation might be solved with MRI positive fiducial markers. The latter two limitations can hopefully be solved in near future with metallic artifact reduction MRI sequences and increase of practical FOV size for the scanners. There have been studies regarding the automatic seed verification, but according to the recently published articles the most reliable method for gold seed identification is visual observation by a clinical personnel [Citation23,Citation24]. It is likely that future studies will introduce methods enabling MRI-only based RT planning for the majority of the patients. Application of MRI-only workflow in clinics is a new concept for RT community. Our experiences have been gathered since we started MRI-only workflow in the end of 2012. Recently, few other clinics also have implemented such workflow and many clinics are developing sCT methods and are also having clinical trials for MRI-only workflow. For example, Tyagi et al. and Persson et al. evaluated that prostate MRI-only protocol can work in their clinics for 88 and 85% of patients, respectively, that are nearly in similar success level with our experiences (92%) [Citation18,Citation19,Citation25,Citation26]. The success rate differences might be caused primarily by the gold marker visibility in the applied MR images and due to the use of different sCT methods.
One current restriction in clinical routine is the lack of support of MRI-only workflow in most treatment planning software, requiring a few extra steps in image handling as described before. However, there are many real benefits of MRI-only workflow. As the planning CT imaging was omitted, the scanner time and personnel resources were saved. For each patient this approach saved one scanning procedure, some waiting time and even some unnecessary exposure to ionizing radiation, although the significance of the last factor is negligible compared to the later exposure of therapeutic radiation.
The most obvious benefit of MRI-only workflow is the elimination of registration errors between image sets from different CT/MRI modalities as the different MRI sequences + the generated sCT are collected during a single imaging session with the same patient set-up. The registration error is a systematic, variable error component that exists for each patient. However, the potential of eliminating this error was not utilized or investigated in this early analysis where the volume of whole prostate was treated with conventional dosages and margins. The registration errors may play more important role in the future if boosting of the cancer specific prostate subvolumes, especially those visible in special MRI sequences, become a clinical standard.
Nowadays more and more radiotherapy departments have full access to an MRI scanner. Many scanner manufacturers provide appropriate accessories for performing treatment planning MRI scans in actual treatment position and with identical patient immobilization opportunities. Under these circumstances the step to the MRI-only treatment planning workflow is not too long. The department needs to maintain geometrical performance of essential MRI sequences at an appropriate level even if the MRI-only planning approach is not applied. Extra hardware is possibly needed for MRI coil supports to cover the entire outer contour of the patient and an MRI compatible laser system for simulation. While most of the commercial TPS vendors do not directly support MRI-only workflow, this can be overcome by an extra step of relative simplistic image handling, like the approach applied by us.
The applied sCT method has worked reliably and robustly by obtaining good agreement of sCT generated ED values corresponding real CT slices [Citation6,Citation13,Citation15,Citation17]. Dosimetric agreement between the applied sCT and CT is within 1%, which is similar to the accuracy as achieved with other high quality sCT methods [Citation6,Citation13,Citation15,Citation17–20,Citation26,Citation27]. The automatic atlas-based bone segmentation works with variety of anatomies and the MR intensity conversion functions well with natural level of MR intensity variations and in the absence of major image artifacts [Citation6,Citation13,Citation15,Citation17]. The sCT method works with different MR scanners, sequences and their parameters [Citation13,Citation15]. However, the method can be sensitive to changes in scanner settings and requires adequate and regular quality assurance. In case of any major changes to MR platform or sequence parameters, the MR to sCT transformation algorithms should be verified and potentially readjusted.
As we have mostly converted the CT based workflow to MRI based process without any changes, no improvements in clinical outcome were expected and indeed they were not observed. No treatment related factor of RT was changed from imaging to treatment and each step of MRI based workflow was tested separately before clinical application against the CT based workflow. The observed differences in step-by-step analysis through the whole RT procedure were related to the small improvement in prostate delineation accuracy in the apex area [Citation22] and elimination of the registration error between CT and MRI sets. The MRI based treatment planning is the first and natural step towards a full MRI based RT workflow from planning to image guidance, where MRI also replaces (CB)CT in image guidance. That is being tested in those centers where the first MRI guided treatment units (cobalt or linacs) are implemented.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Khoo V, Joon D. New developments in MRI for target volume delineation in radiotherapy. Br J Radiol. 2006;79:2–15.
- Sciarra A, Barentsz J, Bjartell A, et al. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. Eur Urol. 2011;59:962–977.
- Dirix P, Haustermans K, Vandecaveye V. The value of magnetic resonance imaging for radiotherapy planning. Semin Radiat Oncol. 2014;24:151–159.
- Expert Panel on Radiation Oncology-Prostate, Zaorsky NG, Showalter TN, et al. CR Appropriateness Criteria® external beam radiation therapy treatment planning for clinically localized prostate cancer, part I of II. Adv Rad Oncol. 2017;2:62–84.
- Kapanen M, Collan J, Beule A, et al. Commissioning of MRI-only based treatment planning procedure for external beam radiotherapy of prostate. Magn Reson Med. 2013;70:127–135.
- Korhonen J, Kapanen M, Keyriläinen J, et al. Influence of MRI-based bone outline definition errors on external radiotherapy dose calculation accuracy in heterogeneous pseudo-CT images of prostate cancer patients. Acta Oncol. 2014;53:1100–1106.
- Mah D, Steckner M, Hanlon A, et al. MRI simulation: effect of gradient distortions on three-dimensional prostate cancer plans. Int J Radiat Oncol Biol Phys. 2002;53:757–765.
- Chen Z, Ma C-M, Paskalev K, et al. Investigation of MR image distortion for radiotherapy treatment planning of prostate cancer. Phys Med Biol. 2006;51:1393–1403.
- Crijns SPM, Bakker CJG, Seebinck PR, et al. Towards inherently distortion-free MR images for image-guided radiotherapy on an MRI accelerator. Phys Med Biol. 2012;57:1349–1358.
- Torfeh T, Hammoud R, Perkins G, et al. Characterization of 3D geometric distortion of magnetic resonance imaging scanners commissioned for radiation therapy planning. Magn Reson Imaging. 2016;34:645–653.
- Gustafsson C, Nordström F, Persson E, et al. Assessment of dosimetric impact of system specific geometric distortion in an MRI only based radiotherapy workflow for prostate. Phys Med Biol. 2017;62:2976–2989.
- Lee Y, Bollet M, Charles-Edwards G, et al. Radiotherapy treatment planning of prostate cancer using magnetic resonance imaging alone. Radiother Oncol. 2003;66:203–216.
- Koivula L, Kapanen M, Seppälä T, et al. Intensity-based dual model method for generation of synthetic CT images from standard T2-weighted MR images - generalized technique for four different MR scanners. Radiother Oncol. 2017;125:411–419.
- Johansson A, Karlsson M, Nyholm T. CT substitute derived from MRI sequences with ultrashort echo time. Med Phys. 2011;38: 2708–2714.
- Korhonen J, Kapanen M, Keyriläinen J, et al. A dual model HU conversion from MRI intensity values within and outside of bone segment for MRI-based radiotherapy treatment planning of prostate cancer. Med Phys. 2014;41:1–13.
- Kapanen M, Tenhunen M. T1/T2*-weighted MRI provides clinically relevant pseudo-CT density data from pelvic bones in MRI-only based radiotherapy treatment planning. Acta Oncol. 2013;52: 612–618.
- Koivula L, Wee L, Korhonen J. Feasibility of MRI-only treatment planning for proton therapy in brain and prostate cancers: Dose calculation accuracy in substitute CT images. Med Phys. 2016;43:4634–4642.
- Edmund JM, Nyholm T. A review of substitute CT generation for MRI-only radiation therapy. Radiat Oncol. 2017;12:28.
- Johnstone E, Wyatt JJ, Henry AM, et al. Systematic review of synthetic computed tomography generation methodologies for use in magnetic resonance imaging-only radiation therapy. Int J Radiat Oncol Biol Phys. 2018;100:199–217.
- Korhonen J, Kapanen M, Keyriläinen J, et al. Absorbed doses behind bones with MR image-based dose calculations for radiotherapy treatment planning. Med Phys. 2013;40:1–10.
- Korhonen J, Kapanen M, Sonke JJ, et al. Feasibility of MRI-based reference images for image-guided radiotherapy in the pelvis with either cone-beam computed tomography or planar localization images. Acta Oncol. 2014;54:1–7.
- Seppälä T, Visapää H, Collan J, et al. Converting from CT- to MRI-only-based target definition in radiotherapy of localized prostate cancer: a comparison between two modalities. Strahlenther Onkol. 2015;191:862–868.
- Gustafsson C, Korhonen J, Persson E, et al. Registration free automatic identification of gold fiducial markers in MRI target delineation images for prostate radiotherapy. Med Phys. 2017;44:5563–5574.
- Ghose S, Mitra J, Rivest-hénault D, et al. MRI-alone radiation therapy planning for prostate cancer: Automatic fiducial marker detection. Med Phys. 2016;43:2218.
- Tyagi N, Fontenla S, Zelefsky M, et al. Clinical workflow for MR-only simulation and planning in prostate. Radiat Oncol. 2017;12:119.
- Persson E, Gustafsson C, Nordström F, et al. MR-OPERA: a multicenter/multivendor validation of magnetic resonance imaging only prostate treatment planning using synthetic computed tomography images. Int J Radiat Oncol Biol Phys. 2017;99:692–700.
- Dowling J, Lambert J, Parker J, et al. An atlas-based electron density mapping method for magnetic resonance imaging (MRI)-alone treatment planning and adaptive MRI-based prostate radiation therapy. Int Radiat Oncol Biol Phys. 2012;83:5–11.
