The last decade has witnessed important advances in the management of metastatic melanoma, even if it remains the deadliest cutaneous cancer. Recent data report an increase in incidence of melanoma in young adults [Citation1], with a more widespread use of adjuvant immunotherapy [Citation2] and targeted treatment also in high risk early disease [Citation3]. Thus, quality of life issues, including fertility have gained more attention. Limited data are available on the reproductive safety of systemic treatments in young patients. Given the increasing use of targeted agents in clinical practice, it is becoming relevant to properly understand if these agents may impact fertility and to ascertain their effects on fetal wellbeing if used in a pregnant patient.
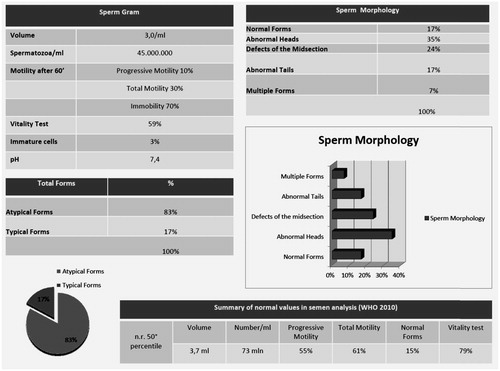
Here, we report the case of a 29-year-old male patient with BRAF V600E mutated metastatic melanoma who fathered while assuming Dabrafenib and Trametinib. After two years of treatment, the patient intentionally had, unprotected intercourse with his wife who eventually got pregnant. Of note, the patient had performed a spermiogram six months before conception, showing only limited alterations in sperm counts and motility that were anyhow within the normal ranges ().
Figure 1. Semen analysis (sperm count, motility, and morphology) performed by patient during dabrafenib and trametinib, and relative normal ranges.
As no data were available at that time about the possible fetal consequences of fathering during Dabrafenib and Trametinib treatment, pregnancy was considered as high risk and intensive monitoring with fetal ultrasound and uterine Doppler examinations.
Pregnancy was uneventful with a normally growing fetus and normal amount of amniotic fluid. At 40 weeks gestation, a healthy 3770 g baby girl was born after spontaneous delivery. The infant had an uncomplicated stay with normal cardiac, renal and lung functions and with blood exams within the normal ranges. Neurological and mental development as measured by the Mental Development Index were normal at 6 and 12 months evaluations. No paternity test was performed.
Approximately 50% of malignant cutaneous melanoma carry a mutation in the BRAF proto-oncogene, which is involved also in fetal cardio-facial-cutaneous development [Citation4].
Biologically, BRAF gene encodes a protein belonging to the raf/mil family of serine/threonine protein kinases [Citation5]. The protein plays a significant role in regulating the RAS/RAF/MEK/ERK (MAPK) signaling pathway, which affects cell division, differentiation, secretion and all major developmental processes before mid-gestation (gastrulation, neurulation, turning, and early organogenesis) [Citation6]. BRAF/MEK expression in normal human tissues was found in germ, interstitial, Leydig testis cells as well as in extraembryonic tissues such as trophoblasts cells and placenta [Citation7]. Dabrafenib, a selective mutant-BRAF inhibitor, and Trametinib, a MEK inhibitor, were both approved by FDA and EMA as combination therapy for BRAF V600 mutated metastatic or unresectable melanoma [Citation8].
Dabrafenib and Trametinib are both FDA pregnancy category D drugs. Dabrafenib is known to be teratogenic and embryotoxic in rats when used at doses three times the human exposure at the recommended clinical dose. In particular, studies on rats and dogs using repeated doses of dabrafenib (≥0.2 times as compared with the human clinical exposure based on AUC), showed significant testicular degeneration/depletion which was still present following a four-week recovery period. Therefore, preclinical studies demonstrated that Dabrafenib may impair fertility in males and cause fetal harm by interfering with BRAF physiological function, which is essential for the developing embryo. Speculatively, the BRAF/MEK inhibitors act by inhibiting only the mutated forms of the genes involved on MAPK pathway, while on healthy cells, including spermatogonial cells, they could have paradoxical effects. In fact, although there are no known effects induced by simultaneous BRAF/MEK inhibition on spermatogenesis nor demonstrated consequences at the level of spermatogonia, we cannot exclude an induced mutagenesis on germ line cells similar to that observed with chemotherapies. There is only one case report of a 37-year-old woman with metastatic melanoma who got pregnant during treatment with the BRAF inhibitor Vemurafenib. Despite a pronounced antitumoral treatment effect, the fetal growth curve was impacted with a constantly reduced growth parameters during gestation. The patient stopped vemurafenib during the last three months of pregnancy and delivered a 1028 g healthy newborn infant at 30 weeks of gestation [Citation9].
To our knowledge, this is the first observation showing that combined Dabrafenib and Trametinib treatment did not cause any mutagenic effect on spermatogonia or ineffective spermatogenesis. Despite studies on the effects of new targeted agents on male reproductive system are limited [Citation10], we have some data on Imatinib, a tyrosine kinase inhibitor of the Bcr-Abl oncogene, KIT-receptor, and PDGF-receptor. Imatinib treatment of adult rodents has only shown modest effects on spermatogenesis or male fertility, mainly through endocrine mechanisms. One study with Imatinib reported a decline in sperm count to <1 million/ml although another study reported normal sperm counts [Citation11].
Moreover, lessons from studies of gene knockouts in mice should warn us about detrimental effects on male fertility through complete inhibition of the gene products also during targeted therapies [Citation12]. Therefore, it could be important to test biomarkers of male fertility, preferably sperm count and quality together with testosterone levels, in patients receiving these new agents to ascertain their effects on male reproductive function.
Until data on this potential interaction will not be available, effective, double method contraception should be used by patients receiving target therapies as well as their partners. For male patients who are scheduled for long-term treatments that may affect their chance of childbearing, sperm banking should be planned before treatment initiation. Young women desiring future fertility should also be counseled on available fertility preserving options before starting target therapies.
In conclusion, even if this is the only report available, Dabrafenib and Trametinib appear to mildly influence spermatogenesis, without negatively impact on pregnancy and on embryo/fetal development. If pregnancy occurs, embryo development should be monitored with standard procedures.
Disclosure statement
Emilia Cocorocchio: Honoraria from BMS, Novartis.
Laura Pala: Honoraria from Novartis.
Pier Francesco Ferrucci: Honoraria from Novartis, BMS, Pierre Fabre.
References
- Erdmann F, Lortet-Tieulent J, Schuz J, et al. International trends in the incidence of malignant melanoma 1953–2008 – are recent generations at higher or lower risk? Int J Cancer. 2013;132:385–400.
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835.
- Amaria RN, Prieto PA, Tetzlaff MT, et al. Neoadjuvant plus adjuvant dabrafenib and trametinib versus standard of care in patients with high-risk, surgically resectable melanoma: a single-centre, open-label, randomised, phase 2 trial. Lancet Oncol. 2018;19:181–193.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147.
- Cohen C, Zavala-Pompa A, Sequeira JH, et al. Mitogen-activated protein kinase activation is an early event in melanoma progression. Clin Cancer Res. 2002;8:3728–3733.
- Wojnowski L, Stancato LF, Larner AC, et al. Overlapping and specific functions of Braf and Craf-1 proto-oncogenes during mouse embryogenesis. Mech Dev. 2000;91:97–104.
- Weizmann Institute of Science. GeneCards: The Human Gene Database. [cited 2018 Jan 10]. Available from: http://www.genecards.org/cgibin/carddisp.pl?gene=BRAF.
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703.
- Maleka A, Enblad G, Sjörs G, et al. Treatment of metastatic malignant melanoma with vemurafenib during pregnancy. J Clin Oncol. 2013;31:10.
- Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013;100:1180–1186.
- Shash E, Bassi S, Cocorocchio E, et al. Fatherhood during imatinib. Acta Oncol. 2011;50:734–735.
- Schultheis B, Nijmeijer BA, Yin H, et al. Imatinib mesylate at therapeutic doses has no impact on folliculogenesis or spermatogenesis in a leukaemic mouse model. Leuk Res. 2012;36:271–274.
