Abstract
Electrochemotherapy is now in routine clinical use to treat cutaneous metastases of any histology, and is listed in national and international guidelines for cutaneous metastases and primary skin cancer. Electrochemotherapy is used by dermatologists, surgeons, and oncologists, and for different degrees and manifestations of metastases to skin and primary skin tumours not amenable to surgery. This treatment utilises electric pulses to permeabilize cell membranes in tumours, thus allowing a dramatic increase of the cytotoxicity of anti-cancer agents. Response rates, often after only one treatment, are very high across all tumour types. The most frequent indications are cutaneous metastases from malignant melanoma and breast cancer. In 2006, standard operating procedures (SOPs) were written for this novel technology, greatly facilitating introduction and dissemination of the therapy. Since then considerable experience has been obtained treating a wider range of tumour histologies and increasing size of tumours which was not originally thought possible. A pan-European expert panel drawn from a range of disciplines from dermatology, general surgery, head and neck surgery, plastic surgery, and oncology met to form a consensus opinion to update the SOPs based on the experience obtained. This paper contains these updated recommendations for indications for electrochemotherapy, pre-treatment information and evaluation, treatment choices, as well as follow-up.
Introduction
Electrochemotherapy established its position as treatment for cutaneous primary and secondary tumours [Citation1–3]. The practice of electrochemotherapy has developed through clinical trials [Citation4–13], the first standard operating procedure (SOP) for electrochemotherapy of cutaneous tumours [Citation14], as well as the clinically certified electric pulse generator, to a point where electrochemotherapy is routinely used.
The first SOP was prepared for the treatment of cutaneous tumours of less than 3 cm in diameter [Citation6,Citation14]. Since electrochemotherapy worked extremely well for tumours in the small diameter range, studies continued to investigate a role for larger tumours such as large breast cancer chest wall recurrences [Citation9], and currently many centres are treating patients with considerable tumour involvement of the skin. Due to the expanded experience, and keeping in mind the success of the first SOP to help align treatment between centres, there was a need for revision in order to integrate knowledge obtained from the centres routinely using electrochemotherapy, as well as to address the current and expanded use of electrochemotherapy in a larger and more diverse patient population.
The simple and effective principle of electrochemotherapy is based on a physical approach to permeabilize cells in the tissue exposed to electric pulses, in order to enable increased cellular uptake of hydrophilic chemotherapeutics that have hampered transport through the cell membrane. By electroporation, bleomycin cytotoxicity, and to a lesser extent cisplatin cytotoxicity, may be increased dramatically within the area where the electric field has been applied. Therefore, electrochemotherapy has a high therapeutic index, i.e., significantly increased action on treated tumours and very limited side effects on normal tissues. Currently bleomycin is by far the most utilized drug for electrochemotherapy [Citation8–10,Citation12,Citation13,Citation15–18], and intravenous infusion is the most common administration of the drug [Citation8,Citation9,Citation12,Citation13].
Due to the dramatic increase in cytotoxicity, electrochemotherapy is effective in all solid tumours, as reported so far; melanoma, adenocarcinoma (breast or other), basal cell carcinoma, squamous cell carcinoma, sarcomas, and other solid tumour malignancies. Electrochemotherapy is a local treatment, and after drug administration the treatment area must be uniformly covered by an adequate electric field, a consideration similar to surgical approaches. Throughout the published literature remarkably consistent responses have been reported, with an approximately 80% OR (objective response rate) including all tumour histologies, and approximately 60–70% CR (complete remission) rate after once-only treatment [Citation6,Citation8,Citation10,Citation12,Citation13,Citation15–17,Citation19,Citation20]. These results were demonstrated on tumours smaller than 3 cm in diameter after single electrochemotherapy session, and although bigger tumours have somewhat lower response rates, these remain high [Citation8,Citation9,Citation11,Citation21]. Electrochemotherapy may be repeated, in case of progression or in cases where tumour remains.
The basic mechanism of action is permeabilization of tumour cells, allowing passage over the cell membrane and thus a direct cytotoxic effect of bleomycin or cisplatin. However there are also anti-vascular effects of electrochemotherapy which can contribute substantially, especially in well vascularized tumours [Citation10,Citation22]. This effect is dual, as application of electric pulses causes vasoconstriction, inducing drug entrapment (termed the vascular lock), and thereafter vascular disruption, due to the endothelial cell death in the tumour vessels [Citation23–25]. Furthermore, in line with tumour cell destruction also local immune response may contribute to overall anti-tumour effectiveness of electrochemotherapy [Citation26–31].
Electrochemotherapy for internal tumours, such as liver, pancreas and lung, is currently being investigated in a number of clinical trials, but falls outside the scope of the current revision of the SOPs which pertain to the treatment of cutaneous tumours.
Electrochemotherapy is currently in use in many cancer centres, and is used by dermatologists, surgeons, and oncologists, in agreement with national and international guidelines for the treatment of metastases to the skin, and primary skin cancer [Citation1,Citation2,Citation32]. The current SOP update takes into account the considerable experience gained, the expansion of indications due to the robustness of the technology, as well as the diversity of patients treated with electrochemotherapy.
In order to produce the revised SOP we assembled authors of the original SOP, along with a cross-disciplinary team of dermatologists, plastic surgeons, head and neck surgeons, general surgeons, and oncologists, all highly experienced in the use of electrochemotherapy for cutaneous tumours, representing institutions across Europe. Below, all important steps, from patient referral and indications over treatment to follow-up are described.
Patient referral and indications for electrochemotherapy
Patients should be referred after multidisciplinary team discussion (MDT) and must be advised about treatment alternatives. It must be evaluated whether, given the localization and extent of tumour, the patient is likely to benefit from electrochemotherapy. The patients’ general condition and expected survival should be weighed against the benefit expected from treatment. Last but not least it is important to define the purpose of the treatment together with the patient and to match his or her expectations. Patients referred for electrochemotherapy present with quite diverse clinical manifestations of cutaneous metastases or primary skin tumours; from the patient with hundreds of very small metastases, to patients with single or few metastases and to patients with very large, ulcerated tumours.
Indications for treatment include:
Cutaneous metastases, of any histology, which are symptomatic due to bleeding, ulceration, oozing, odour, or pain.
Progression of cutaneous metastases, where development of symptoms as listed above, is expected.
Primary skin cancers, including recurrent tumours, where other treatment modalities (surgery, radiotherapy, and systemic therapies) have failed or are not possible.
Patients who are receiving systemic therapy, but where cutaneous metastases are progressing or not responding despite satisfactory response to systemic therapy in internal organs.
Patient preference for electrochemotherapy, after other treatment possibilities have been thoroughly explained to the patient.
Contraindications
Pregnancy, lactation: Potentially fertile patients must use adequate contraception.
Allergy or hypersensitivity to bleomycin or cisplatin: Lifetime dose of bleomycin should not exceed 400,000 International Units (IU).
Pre-treatment examinations
Medical history and pre-treatment considerations
Medical history should be examined, and special attention should be paid to the following:
Previous problems with respect to local anaesthesia (lidocaine) or general anaesthesia.
Lung function (relevant when bleomycin administration intravenously is planned): A pre-treatment lung diffusion capacity test is recommended only if the patient has symptomatic pulmonary disease. For patients with benign tumours (e.g., vascular malformations and keloids), a lung diffusion capacity test should be performed before and after treatment.
Renal function: creatinine should be <150 µm/l to ensure adequate renal clearance when using intravenous bleomycin. In cases of low renal clearance, a lower administered dose of bleomycin must be considered for intravenous use, level of reduction according to institutional or national guidelines. Furthermore intratumoural administration can be a good alternative.
Coagulation abnormalities do not preclude treatment, but may be corrected to pre-surgery standards for the institution. Likewise medication decreasing coagulation is not a contradiction, but may be handled according to usual procedure for surgery.
Liver disease does not preclude the use of electrochemotherapy.
Pre-treatment ECG analysis is needed as per anaesthetic indications only.
The presence of a pacemaker is not a contra-indication to electrochemotherapy even in close proximity as long as direct contact with the pacemaker is avoided. When treating close to a defibrillator it should be disconnected before applying the pulses and reconnected immediately thereafter.
Patients with pre-existing pain problems may experience exacerbation after electrochemotherapy, and therefore examination of need for improved pain control is recommended before electrochemotherapy [Citation33].
Combination with other treatments. Progression in cutaneous metastases in patients who are experiencing stable disease in internal organs on present anti-neoplastic treatment is relatively common, possibly due to lower drug concentrations in skin, in particular in hypo-perfused pre-irradiated areas. In these cases electrochemotherapy may be performed either concomitantly (as with endocrine treatment), or allowing one week before, respectively one day after electrochemotherapy spacing to other systemic treatment (chemotherapy, targeted therapy), with adequate thrombocyte and leukocyte function at the day of electrochemotherapy. Combination with immunotherapy is currently being investigated, in particular electrochemotherapy with check-point inhibitors [Citation34,Citation35].
Presence of symptoms to be alleviated by electrochemotherapy:
Bleeding
Oozing
Pain
Bulky tumour
Odour
Assessing size and number of tumours
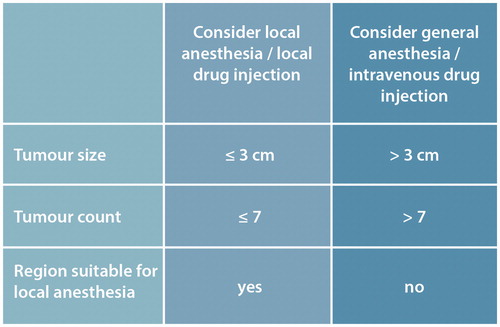
The size of largest tumours and number of tumours must be assessed to determine treatment strategy (). With very large tumours (exophytic more than 3 cm thick) consider debulking the exophytic element within the electrochemotherapy session.
With current equipment, and using a 40 min treatment window, investigators have treated very large lesions up to 30 × 40 cm in extension in one session [Citation9] and experience reported by authors of this paper.
Documentation and imaging
Photographic documentation to document the size and location of the tumours must be done before treatment as a baseline in order to monitor treatment efficacy. A ruler should be present in the photo and photos should always be done in the same angle and perspective (, [Citation36]). A recommendation would be to report sizes of e.g., up to seven tumour nodules, including the largest, in order to have a reasonable baseline assessment.
Figure 2. Patients referred for electrochemotherapy present with different clinical stages of cutaneous metastasis. Evaluation of smaller tumours may favourably be performed using digital photography and ruler (top panel, from [Citation36]). For larger tumours, imaging such as PET/CT can be very valuable in determining response (bottom panel, from [Citation9]).
![Figure 2. Patients referred for electrochemotherapy present with different clinical stages of cutaneous metastasis. Evaluation of smaller tumours may favourably be performed using digital photography and ruler (top panel, from [Citation36]). For larger tumours, imaging such as PET/CT can be very valuable in determining response (bottom panel, from [Citation9]).](/cms/asset/b73d2438-41fb-4803-b070-d3f683dca59d/ionc_a_1454602_f0002_c.jpg)
Other imaging modalities such as MRI, CT, PET for documentation of effect [Citation37] (), and ultrasound may be used for guidance.
Patient information
Patients must be informed of expected outcomes of treatment, as well as side-effects associated with treatment, both in writing and oral communication. Topics covered must include (1) a small risk of lung fibrosis associated with iv bleomycin administration, (2) risk of post-procedure pain (which may be counteracted by pain management plans, as well as predicted before treatment), (3) expected duration and nursing management plan for ulcerated lesions, (4) risk of hyperpigmentation in the skin after use of bleomycin.
Treatment
Anaesthesia
Patients treated by electrochemotherapy need some form of anaesthesia for the management and alleviation of symptoms related to application of needle electrodes as well as the electric pulse delivery. The regimen of anaesthesia is determined by the clinician/anaesthetist from the following choices ().
Local anaesthesia
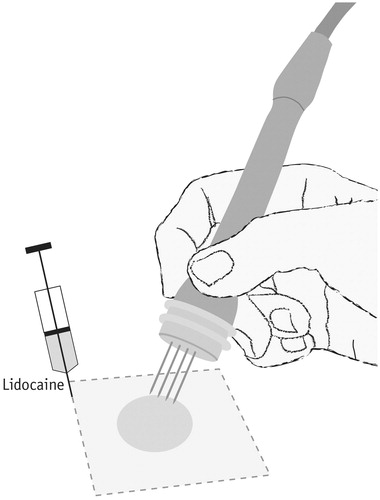
Local anaesthesia may be performed as a rectangular infiltration of local anaesthetic around the area to be treated, by injecting along four lines so that the nodule is ‘fenced in’ by local anaesthetic. Be sure that an even amount of anaesthetic is injected along all four sides of the rectangle, so that pain transmission is completely blocked. Be sure to include a margin around the area to be treated of 0.5–1 cm. If more than one nodule is to be treated, it is necessary to consider the total amount of lidocaine: For example, in a 70 kg patient the maximal dose without epinephrine is 210 mg (21 ml of 1% lidocaine or 10.5 ml of 2% lidocaine) and with epinephrine 420 mg (42 ml of 1% or 21 ml of 2%). This will set the maximum of nodules treatable by one session with local anaesthesia, not surpassing 3 mg/kg of lidocaine without epinephrine or 6 mg/kg for lidocaine with epinephrine. Another possibility is to use tumescent local anaesthesia, where a higher volume of lower concentration local anaesthesia is used to infiltrate the treatment area [Citation38].
If it is anticipated that there will be less than 0.5 cm from the tip of the electrodes to periosteum, the option of local anaesthesia should be reconsidered, since it may be difficult to achieve suitable analgesia with local anaesthetic alone. Another potential restriction to local anaesthesia is the localization of the nodules in previously irradiated areas: experience has shown that is seems more difficult to apply local anaesthesia in these previously irradiated areas and it is recommended to administer more lidocaine, as well as allow more time for diffusion due to fibrosis in these tissues. Small cutaneous tumours may be anaesthetized by local infiltration of lidocaine just below the tumour, however, still covering the area of electroporation. This may increase the number of small nodules that can be treated with local anaesthetic in one session.
Contractions of underlying musculature will appear in the majority of treatments, due to a direct effect of the pulse. The contraction can be alleviated by lifting the treatment area up, using a gauze pad. It must be explained to the patient that these brief contractions will occur.
Spinal anaesthesia or regional anaesthesia
Spinal anaesthesia may be considered for treatment of lower limbs, and regional anaesthesia can be considered, when lesions to be treated are confined to one limb.
Sedation
Premedication with sedatives is not necessary, and many centres do not use it. There are, however, other centres which feel that sedation in connection with local anaesthesia works well [Citation18].
General anaesthesia
General anaesthesia is performed in accordance with guidelines and preferences of the treating institution. Propofol sedation is a preferred choice because the procedure is brief. Relaxation is performed in some centres, in order to avoid contractions of underlying musculature, but many treating physicians feel that this is unnecessary. However, patients being treated on the upper thorax or head and neck area need intubation in order to avoid muscle contractions influencing the placement of e.g., a larynx-mask.
Before administering bleomycin FiO2 must be max 30% to reduce risk of bleomycin associated lung toxicity. After anaesthesia, oxygen administration should be limited to 2 l/min. These are precautions standardly used to reduce risk of toxicity, however this precaution must not be interpreted in a way that the patient is not administered necessary oxygen in case of need.
Drug administration
Bleomycin is either administered intratumourally or intravenously. The potency of bleomycin is measured in units of antimicrobial activity. One unit (U) contains 0.56–0.66 mg of bleomycin and 1 unit (U) is equivalent to 1000 international units (IU) [Citation39]. In Europe bleomycin is either dosed in mg or International Units (IU), whereas Units (U) is the term used in the US.
Intratumoural drug administration is recommended for smaller and fewer tumours, whereas intravenous administration is preferable for multiple and larger tumours ().
Intravenous route: Measure patient height and weight in order to calculate the body surface area (BSA). Intravenous bleomycin is administered at a dose of 15,000 IU/m2 BSA, maximum dose capped at 30,000 IU (corresponding to 2.0 m2 BSA), and modulation due to high age and or compromised creatinine clearance may be performed according to institutional guidelines. Bleomycin is infused over short time (2–5 min), and 8 min after the completion of infusion, the drug has diffused into tumour tissues and the electric pulses can be applied. A previous recommendation to end treatment at 20 min (equalling 28 min after completion of bleomycin administration) is now expanded to a treatment window of 40 min, based on clinical experience. A recent pharmacokinetic study supports this [Citation40]. It is even seen as possible to continue administering pulses after this time window, if deemed in the interest of the patient to hope for a treatment effect which may indeed be present also after the 40 min window.
Intratumoural route: Measure the lesions to calculate the volumes and concentrations of bleomycin that will have to be prepared (). The recommended concentration of bleomycin injection solution is 1000 IU/ml. Guidance for injection volumes are provided in , although injection can be performed by approximation making sure the entire tumour volume is covered. The upper limit for the bleomycin dose used intratumourally is the dose that would be used if intravenously administrated. When using cisplatin the recommended mixture is 1 mg/ml, and the recommended injection dose is to inject to fill the tumour volume with drug.
Choice of electrode
Choose the appropriate electrode (the electric generator must be able to adapt to various geometries in order to supply the correct field). If the tumour is less than 3 cm, consider using parallel needle array electrodes. When larger areas need to be covered consider using the hexagonal geometry needle electrodes (). Adjustable needle electrodes (which can be either linear array or hexagonal geometry) allow good support for the needles so that a depth up to 3 cm can be treated, and also so that needle length may be adjusted to need. Finger electrodes can be convenient to use for mucosal tumours as they are more flexible to work with. Plate electrodes may be used for small superficial tumours. In a few cases using more than one type of electrode may be a good solution to cover the tumour correctly.
Figure 4. Different electrodes suit different clinical challenges. Top left; linear array electrode, ideal for smaller tumours. Due to the relatively lower applied voltage (due to small electrode distance), ideal for treatment in local anaesthesia. Hyperpigmentation using this electrode is very little to negligible, and this electrode is thus recommended for use in the facial region. Top right; hexagonal electrode. This electrode should only be used under general anaesthesia, and will often cause hyperpigmentation. The relatively larger area covered allows larger tumours to be treated, making this electrode a good choice for large cutaneous metastases, e.g., chest wall recurrence after breast cancer. Middle left panel; plate electrodes, may be used for exophytic tumours. Middle right panel; finger electrodes, may be used for mucosal tumours in e.g., the oral cavity. Bottom; Adjustable electrodes allow better support for the needles, and allows adjustment for tumours with heterogeneous size.

examplifies treatment procedure. After the pulses are delivered the electrodes are repositioned in order to cover the whole tumour. The electric field drops off very quickly outside the area of the electrodes, so the area to be treated by pulses must be encompassed within the electrodes. Therefore, not only must the appropriate electrode be chosen, but the pattern of applications necessary to cover the tumour volume must be decided as well. Overlap is recommended in the tumour tissue to ensure sufficient coverage. When treating large tumours adjacent to large vessels, ultrasound guidance can be employed.
The linear electrode may give better cosmetic results (due to lower applied voltage as a function of smaller electrode size), and is recommended when treating the facial area.
The hexagonal electrode will be able to cover larger areas.
Be sure to cover the entire treatment area with a margin.
Avoid excessive treatment (too much overlap) in normal tissue.
Figure 5. Carrying out treatment. Electrochemotherapy is performed under analgesia; for smaller tumours local anaesthesia (as indicated here) whereas larger tumor conglomerates necessitate general anaesthesia. The chemotherapeutic agent, bleomycin, is either injected locally (few, small tumours), or systemically for large and/or many tumours. After drug administration the electric pulses are administered through the electrodes that are inserted into the tumour tissue. The electrodes are systematically moved, and pulses applied again, in order to cover the entire tumour area. The pulsing sequence is very brief, and therefore even large tumours may be covered in the course of a single treatment because the procedure proceeds quickly.
Other considerations
Antibiotics: Administration of antibiotics is advised (choice of antibiotic according to institutional guidelines) peri-operatively, for patients with ulcerated lesions.
Pain management: The majority of patients have low pain scores both before and after electrochemotherapy [Citation33], but a subset of patients experience more serious pain after treatment. It has been shown that patients at risk for pain after electrochemotherapy may be identified at the first visit by predictors, of which the most important is a high pain score before treatment, but also previous irradiation and large tumours are predictors of post-procedure pain [Citation33]. shows recommendations for pain management to be instituted at the pre-treatment visit.
Figure 6. Pain management plan. Most patients treated with electrochemotherapy only experience no or limited pain after treatment. A subset of patients do, however, experience pain and these patients may be predicted from their pain score before treatment [Citation33], i.e., at the pre-treatment visit. Based on patient pain score (NRS = numeric rating scale, where 0 is no pain at all, and 10 is the worst pain imaginable), the patient may be assessed at the pre-treatment visit for a pain management plan. Other factors that may forewarn higher risk of post-procedure pain are previous irradiation and large tumours [Citation33].
![Figure 6. Pain management plan. Most patients treated with electrochemotherapy only experience no or limited pain after treatment. A subset of patients do, however, experience pain and these patients may be predicted from their pain score before treatment [Citation33], i.e., at the pre-treatment visit. Based on patient pain score (NRS = numeric rating scale, where 0 is no pain at all, and 10 is the worst pain imaginable), the patient may be assessed at the pre-treatment visit for a pain management plan. Other factors that may forewarn higher risk of post-procedure pain are previous irradiation and large tumours [Citation33].](/cms/asset/a7d68f7a-b473-43e4-a9df-a9489ac651ae/ionc_a_1454602_f0006_c.jpg)
Wound care and nursing considerations
When treating with intravenous doses of bleomycin, hyperpigmentation may occur in the skin where physical pressure has been exerted. This includes marks from needle electrodes, but also skin stretching due to removal of ECG-electrodes, surgical drapes, or pressure exerted due to the position on the surgical bed may induce hyperpigmentation. Therefore, attention to positioning, and gentle removal of tags and drapes after surgery is important.
Lesions can be covered with non-adherent dressings.
For ulcerated lesions alginates, medical honey, silver or charcoal may be applied underneath.
Debridement of necrotic tissue, as treatment effects set in, is important.
There is no limitation in showering after treatment.
In infected wounds consider using antibiotics.
Pain management according to guidelines (, [Citation33]).
Patients with cutaneous metastases often experience distress due to disfigurement, oozing and bleeding, as well as the feeling of cancer stigmatisation. Thus special consideration pertaining to the psychological challenges associated with cutaneous metastases is important.
Specific dedication and training for treatment of this patient group is important.
Follow-up and retreatment
Follow-up is planned individually depending on patient need. Follow-up schedule of two weeks (possibly nurse consultation or telephone consultation), one month, two months, four months and six months is frequently used as guideline. Evaluation is done with measurement of tumour size and documented with photography. For larger lesions longer healing time will be necessary () and longer follow-up and nursing may be necessary. Pain is managed based on patient report of pain ().
Patients can be retreated if the tumour is not sufficiently treated at the first session or if recurrence appears within or outside the treated area. It is suggested to discontinue if lung diffusion capacity decreases with more than 25% from baseline. There is no data regarding minimal interval between retreatments, but at least four weeks is recommended when using intravenous bleomycin. Maximum therapeutic effect is seen after six to eight weeks. For intratumoural drug application further treatments can be applied whenever needed .
Access to information
In addition to the written information provided to the patient at the first visit it is also recommended that information is made available, e.g., at the hospital website, for care providers at home.
Use of electrochemotherapy in clinical practice
As electrochemotherapy is efficient with limited side effects for cutaneous tumours, and treatment can be performed over one or few sessions, the procedure is now increasingly being used, and also introduced in to clinical guidelines. This includes guidelines for advanced melanoma [Citation41], and primary squamous cell carcinoma [Citation32], as well as guidance for treatment of cutaneous metastases and primary skin tumours [Citation1,Citation2].
Recent studies in respectively cutaneous and mucosal tumours in the head and neck region [Citation13,Citation42], also based on the treatment guidelines here described, have shown promising results with an overall response rate of 56% in previously treated patients with recurrent mucosal head and neck cancer [Citation42]. Specifically elaborated guidelines for this indication may be anticipated.
Furthermore, electrochemotherapy is now being considered for a number of indications for tumours in internal organs, in clinical trials.
The present updated SOP for the treatment of cutaneous tumours is published to provide guidelines for the treatment of primary and metastatic tumours of the skin, based on broad experience across treatment centres and medical specialities.
Acknowledgments
This paper has been written solely by the authors.
Disclosure statement
JG is an inventor of patents (however not regarding the topics covered in this paper) licensed to IGEA and has been invited to lecture at meetings on electrochemotherapy by IGEA. LMM is an inventor of CNRS patents related to cliniporator equipment and has paid consultancy for IGEA. CK received honoraria for lectures and was invited to meetings on electrochemotherapy by IGEA. Travel cost for one meeting regarding this work has been sponsored by IGEA.
References
- National Institute for Health and Care Excellence (NICE). Electrochemotherapy for primary basal cell carcinoma and primary squamous cell carcinoma; [Internet] 2014. Available from: www.nice.org.uk.
- National Institute for Health and Care Excellence (NICE). Electrochemotherapy for metastases in the skin from tumours of non-skin origin and melanoma; 2013. Available from: http://publicationsniceorguk/electrochemotherapy-for-metastases-in-the-skin-from-tumours-of-non-skin-origin-and-melanoma-ipg446.
- Kee S, Gehl J, Lee E. Clinical Aspects of Electroporation. New York (NY): Springer; 2011.
- Belehradek M, Domenge C, Luboinski B, et al. Electrochemotherapy, a new antitumor treatment. First clinical phase I–II trial. Cancer. 1993;72:3694–3700.
- Heller R, Jaroszeski MJ, Reintgen DS, et al. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer. 1998;83:148–157.
- Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy – an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Ejc Supplements. 2006;4:3–13.
- Campana LG, Mocellin S, Basso M, et al. Bleomycin-based electrochemotherapy: clinical outcome from a single institution's experience with 52 patients. AnnSurgOncol. 2009;16:191–199.
- Matthiessen LW, Chalmers RL, Sainsbury DC, et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50:621–629.
- Matthiessen LW, Johannesen HH, Hendel HW, et al. Electrochemotherapy for large cutaneous recurrence of breast cancer: a phase II clinical trial. Acta Oncol. 2012;51:713–721.
- Curatolo P, Quaglino P, Marenco F, et al. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Ann Surg Oncol. 2012;19:192–198.
- Campana LG, Valpione S, Mocellin S, et al. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. 2012;99:821–830.
- Campana LG, Valpione S, Falci C, et al. The activity and safety of electrochemotherapy in persistent chest wall recurrence from breast cancer after mastectomy: a phase-II study. Breast Cancer Res Treat. 2012;134:1169–1178.
- Bertino G, Sersa G, De Terlizzi F, et al. European research on electrochemotherapy in head and neck cancer (EURECA) project: results of the treatment of skin cancer. Eur J Cancer. 2016;63:41–52.
- Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur J Cancer Suppl. 2006;4:14–25.
- Campana LG, Testori A, Mozzillo N, et al. Treatment of metastatic melanoma with electrochemotherapy. J Surg Oncol. 2014;109:301–307.
- Curatolo P, Miraglia E, Rotunno R, et al. Electrochemotherapy: a valid treatment for Gorlin-Goltz syndrome. Acta Dermatovenerol Croat. 2013;21:132–133.
- Quaglino P, Mortera C, Osella-Abate S, et al. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. AnnSurgOncol. 2008;15:2215–2222.
- Campana LG, Testori A, Curatolo P, et al. Treatment efficacy with electrochemotherapy: a multi-institutional prospective observational study on 376 patients with superficial tumors. Eur J Surg Oncol. 2016;42:1914–1923.
- Spratt DE, Gordon Spratt EA, Wu S, et al. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol. 2014;32:3144–3155.
- Kunte C, Letule V, Gehl J, et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol. 2017;176:1475–1485.
- Whelan MC, Larkin JO, Collins CG, et al. Effective treatment of an extensive recurrent breast cancer which was refractory to multimodal therapy by multiple applications of electrochemotherapy. Eur J Cancer Suppl. 2006;4:32–34.
- Gehl J, Geertsen PF. Efficient palliation of haemorrhaging malignant melanoma skin metastases by electrochemotherapy. Melanoma Res. 2000;10:585–589.
- Gehl J, Skovsgaard T, Mir LM. Vascular reactions to in vivo electroporation: characterization and consequences for drug and gene delivery. Biochim Biophys Acta. 2002;1569:51–58.
- Markelc B, Sersa G, Cemazar M. Differential mechanisms associated with vascular disrupting action of electrochemotherapy: intravital microscopy on the level of single normal and tumor blood vessels. PLoS One. 2013;8:e59557.
- Sersa G, Jarm T, Kotnik T, et al. Vascular disrupting action of electroporation and electrochemotherapy with bleomycin in murine sarcoma. Br J Cancer. 2008;98:388. 98:
- Andersen MH, Gehl J, Reker S, et al. Dynamic changes of specific T cell responses to melanoma correlate with IL-2 administration. Semin Cancer Biol. 2003;13:449–459.
- Calvet CY, Famin D, Andre FM, et al. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology. 2014;3:e28131.
- Sersa G, Teissie J, Cemazar M, et al. Electrochemotherapy of tumors as in situ vaccination boosted by immunogene electrotransfer. Cancer Immunol Immunother. 2015;64:1315–1327.
- Mozzillo N, Simeone E, Benedetto L, et al. Assessing a novel immuno-oncology-based combination therapy: ipilimumab plus electrochemotherapy. Oncoimmunology. 2015;4:e1008842.
- Calvet CY, Mir LM. The promising alliance of anti-cancer electrochemotherapy with immunotherapy. Cancer Metastasis Rev. 2016;35:165–177.
- Falk H, Lambaa S, Johannesen HH, et al. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma – a case report. Acta Oncol. 2017;56:1126–1131.
- Stratigos A, Garbe C, Lebbe C, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989–2007.
- Quaglino P, Matthiessen LW, Curatolo P, et al. Predicting patients at risk for pain associated with electrochemotherapy. Acta Oncol. 2015;54:298–306.
- Queirolo P, Marincola F, Spagnolo F. Electrochemotherapy for the management of melanoma skin metastasis: a review of the literature and possible combinations with immunotherapy. Arch Dermatol Res. 2014;306:521–526.
- Heppt MV, Eigentler TK, Kahler KC, et al. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis. Cancer Immunol Immunother. 2016;65:951–959.
- Gehl J. [Investigational treatment of cancer using electrochemotherapy, electrochemoimmunotherapy and electro-gene transfer]. Ugeskr Laeger. 2005;167:3156–3159.
- Matthiessen LW, Johannesen HH, Skougaard K, et al. Dual time point imaging fluorine-18 flourodeoxyglucose positron emission tomography for evaluation of large loco-regional recurrences of breast cancer treated with electrochemotherapy. Radiol Oncol. 2013;47:358–365.
- Kendler M, Micheluzzi M, Wetzig T, et al. Electrochemotherapy under tumescent local anesthesia for the treatment of cutaneous metastases. Dermatol Surg. 2013;39:1023–1032.
- Gothelf A, Mir LM, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29:371–387.
- Groselj A, Krzan M, Kosjek T, et al. Bleomycin pharmacokinetics of bolus bleomycin dose in elderly cancer patients treated with electrochemotherapy. Cancer Chemother Pharmacol. 2016;77:939–947.
- Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline – Update 2016. Eur J Cancer. 2016;63:201–217.
- Plaschke CC, Bertino G, McCaul JA, et al. European research on electrochemotherapy in head and neck cancer (EURECA) project: results from the treatment of mucosal cancers. Eur J Cancer. 2017;87:172–181.