Abstract
Purpose: To find out what organs and doses are most relevant for ‘radiation-induced urgency syndrome’ in order to derive the corresponding dose–response relationships as an aid for avoiding the syndrome in the future.
Material and methods: From a larger group of gynecological cancer survivors followed-up 2–14 years, we identified 98 whom had undergone external beam radiation therapy but not brachytherapy and not having a stoma. Of those survivors, 24 developed urgency syndrome. Based on the loading factor from a factor analysis, and symptom frequency, 15 symptoms were weighted together to a score interpreted as the intensity of radiation-induced urgency symptom. On reactivated dose plans, we contoured the small intestine, sigmoid colon and the rectum (separate from the anal-sphincter region) and we exported the dose-volume histograms for each survivor. Dose–response relationships from respective risk organ and urgency syndrome were estimated by fitting the data to the Probit, RS, LKB and gEUD models.
Results: The rectum and sigmoid colon have steep dose–response relationships for urgency syndrome for Probit, RS and LKB. The dose–response parameters for the rectum were D50: 51.3, 51.4, and 51.3 Gy, γ50 = 1.19 for all models, s was 7.0e−09 for RS and n was 9.9 × 107 for LKB. For Sigmoid colon, D50 were 51.6, 51.6, and 51.5 Gy, γ50 were 1.20, 1.25, and 1.27, s was 2.8 for RS and n was 0.079 for LKB.
Conclusions: Primarily the dose to sigmoid colon as well as the rectum is related to urgency syndrome among gynecological cancer survivors. Separate delineation of the rectum and sigmoid colon in order to incorporate the dose–response results may aid in reduction of the incidence of the urgency syndrome.
Introduction
When curing a patient from cancer, unfortunately the ionizing radiation that eliminates malignant cells also may trigger long-lasting pathophysiological processes in the normal tissue. When in the gut wall, such processes ultimately may lead to five different syndromes, an urgency syndrome, excessive mucus discharge, leakage syndrome, excessive gas discharge and blood discharge [Citation1]. Moving forward towards the ultimate aim of curing cancer with restored bowel health, we must hinder the underlying pathophysiological processes to start or limit their duration and reverse the damage they may have caused. To partially or fully prevent radiation-induced urgency syndrome, we must learn much more about the relation of the risk organs to the radiation dose and which risk organ or organs are mostly related to the radiation dose.
Radiation-induced urgency syndrome consists of 15 self-assessed atomized symptoms (). A survivor with the syndrome has loose stools, abdominal bloating, urgency, incontinence, fecal leakage symptoms, defecation into clothing without forewarning, anal pain and abdominal pain. The pathophysiological processes that ultimately leads to a long-lasting urgency syndrome probably includes crypt degeneration and transformation to an end stage of dysfunctional fibrotic tissue. Certainly inflammatory processes are involved, e.g., to hinder recruitment of reserve stem cells from a radio-resistant pool, but it is unclear if such processes last for months, years of even decades. Moreover, we do not know if processes in the rectum, the sigmoid colon and the rest of colon produces different symptoms. The bowel has an impressive capacity to heal itself by crypt fission, during this process one crypt may divide to two crypts with stem cells in the base [Citation1,Citation2]. Prevention implies lowering doses to the normal tissue but we may also learn to enhance this reparative capacity by, e.g., avoiding gut-wall starvation during the radiotherapy with a fiber-rich diet, avoid a dysfunctional microbiota by probiotics and block pathways with drugs [Citation1,Citation2]. Abnormal anorectal function is thought to underlie weakness of the anal sphincter and heightened rectal sensitivity in patients after pelvic radiotherapy for gynecological cancer [Citation3]. From the literature, we know that dose to the rectum is a factor associated with urgency-related symptoms [Citation4–8]. In our previous work, we illustrated that dose to both the sigmoid colon and the rectum is related to the self-assessed atomized symptom ‘defecation into clothing without forewarning’ [Citation9–11]. Possibly, the dose to both sigmoid colon and rectum is also important for the development of urgency syndrome.
Table 1. Factor loadings for the symptoms associated with ‘radiation-induced urgency syndrome’Table Footnotea.
In this study, we classified patients as having urgency syndrome or not and we used this as a response in the dose–response models to find associations between dose to the rectum, sigmoid colon and small intestine and urgency syndrome. In other words, the goal of this study was to examine which organs and doses are most strongly associated with the development of urgency syndrome and to derive the corresponding dose–response relationships. We also fitted the most commonly used normal tissue complication probability (NTCP) models to our data in order to identify whether there is a preferable model. This is important since there is always a discussion about whether there is an optimal NTCP model to use in clinical practice in order to optimize the treatment plan and avoid certain normal tissue complications. The ultimate aim was to facilitate a future prevention of urgency syndrome.
Material and methods
Study design and organs at risk
The study included 98 survivors treated for gynecological cancer, 24 (24%) of whom developed urgency syndrome. The cancer survivors received different treatment combinations of external beam radiation therapy (EBRT), surgery and chemotherapy at the Karolinska University Hospital, Stockholm or the Sahlgrenska University Hospital, Gothenburg during the period 1991–2003 giving a follow-up of 3–12 years. This study was a part of a bigger cohort study which includes 650 irradiated cancer survivors (). Among those 650, we excluded survivors with bowel stoma (3%), the ones for whom we were not able to extract the dose–volume histograms (15%) from the treatment planning system and survivors that received brachytherapy (81%). The survivors were treated for all gynaecological cancers that radiotherapy is indicated; endometrial cancer, cervical cancer, ovarian cancer, vaginal, sarcoma, and fallopian tube cancer.
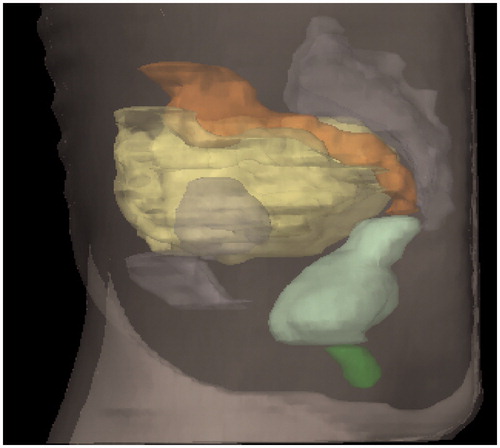
Figure 1. Organs at risk in lateral view: dark green: anal-sphincter; light green: rectum; orange: sigmoid; yellow: small intestines.
The study uses high quality of data that are available due to the standard Swedish practice uniform throughout the country. During the 1990s and 2000s, all patients with cancer in Sweden were treated at clinics based on the geographical region in which they were registered using personal identity numbers. This practice makes it possible for researchers to locate long-term cancer survivors and send postal questionnaires to them.
EBRT was administered according to International Commission on Radiation Units and Measurements (ICRU) 1993 [Citation12] with different linear accelerators with energy 6–50 MV. The treatment techniques used were either two opposing fields or four-field box technique. The fractionation schedule was 1.6, 1.8 or 2.0 Gy per fraction. The treatment was planned based on computed tomography (CT) scanning with the patients in supine position on a flat table top, using laser markers and conversion factors to electron density. The CT slices were 5–20 mm thick. The treatment planning systems used was TMS (Nucletron, Veenendaal, the Netherlands) in Stockholm and Cadplan and Eclipse (Varian Medical Systems, Palo Alto, CA, USA) in Gothenburg.
The prescribed doses were: 40–46 Gy for endometrial cancer, 50 Gy to uterine sarcomas and for cervical cancer similar techniques were used in an initial treatment phase while in a second phase a boost covering a smaller volume to a prescribed total dose of 55–70 Gy, 20 Gy to the whole abdomen and pelvis for ovarian and fallopian tube cancers and an additional 20 Gy to a reduced volume by lowering the cranial margin.
The rectum, the sigmoid colon and small intestine were delineated in the CT scans and the dose–volume histograms were exported for each patient. As we thoroughly described in a previous publication, delineation was performed manually at Karolinska University Hospital, Stockholm and at Sahlgrenska University Hospital, Gothenburg following written instructions and with the guidance of a contouring manual with illustrations [Citation11]. The outer contour of the rectum was delineated, included filling. The rectum was extended from the anal verge to the recto-sigmoid junction. We started delineating the sigmoid colon where the rectum deviates from its midposition to where it turns cranially in the left part of the abdomen connecting to the colon descendens. For the small intestine, we delineated all visible small bowels in the pelvic cavity to the caudal part of the sacroiliac joints [Citation11].
Syndrome as a dose–response outcome
In a previous study of our group a study-specific validated questionnaire was constructed and mailed to all included women [Citation13]. Among the questions in the questionnaire covering bowel health, 28 were included in the factor analysis the result of which led to the description of five distinct radiation-induced survivorship syndromes (urgency syndrome, leakage syndrome, excessive gas discharge, excessive mucus discharge and blood discharge). Urgency syndrome is a combination of 15 different atomized patient-reported bowel symptoms () [Citation1]. To combine the symptoms, factor analyses [Citation1] were used that model the correlations among the studied variables. This is achieved by expressing the variables as linear combinations of a set of unobserved variables called factors. The weights in these linear combinations are called factor loadings and describe the extent to which the variables are associated with the factors. A factor score is a measure of an individual’s position along a factor. To estimate the factor scores, we used a simple linear combination of the estimated factor loadings and the normalized symptom intensities of patient. For a patient to be considered to have urgency syndrome, she should have a factor score greater than the estimated 85th percentile of the patient population.
Dose–response modeling and statistical analyses
To fit the models to the data, maximum likelihood estimation was used. We calculated the dose–volume response parameters using our ‘in house’ software bml, which is based on the software package Nonlinear Programming, Systems Optimization Laboratory (NPSOL) [Citation14]. We estimated dose–response relationships using the Probit model [Citation15] and the dose–volume response parameters using the Relative Seriality (RS) [Citation16] and the Lyman Kutcher Burman (LKB) model [Citation10]. The Lyman model can be combined with any DVH reduction model. In this study, we combined Lyman model with the generalized Equivalent Uniform Dose (gEUD) [Citation17,Citation18]. The gEUD is a way to summarize the whole dose-distribution in a volume of interest to a single figure. The gEUD is defined as the dose that supposedly, if given uniformly to the entire organ, will cause the same complication rate as the true dose distribution.
The response probability P(D) of the organs at risk (OARs) and the syndrome used in the RS model, were calculated using the Probit model. The RS model is parametrized by D50, which is the value of the dose that gives 50% complication probability, γ50 which is the normalized dose–response gradient and s, which is the volume parameter. The LKB model is parametrized by D50, m which is inversely related to the slope of the dose–response curve or the normalized dose–response gradient γ50 () and n which is the volume parameter. The n parameter is inversely related with α parameter of g EUD model (
. To estimate the dose–volume response parameters, α/β was set as a free parameter for all models and OARs. The ratio of α/β is equal to a dose α/β, where the linear and quadratic components of cell killing of linear quadratic equation are equal [Citation19]. The log likelihood (LL) and the Akaike information criterion (AIC) were used as measure of the goodness of fit of the models. The LL assesses the agreement between the measured and predicted by the model results. In AIC method, the LL value is adjusted for the number of parameters of each model. 68% confidence intervals (CI) were also calculated for the model parameters. We used ROC curve analyses in order to identify which organs at risk (dataset) is most strongly correlated with the clinical outcome. The Pearson correlation coefficient r were calculated in order to investigate the volume parameters.
Exploratory factor analysis was performed based on the Spearman correlations between the 28 symptoms. The model parameters were estimated using maximum likelihood estimation. The number of factors was assessed using parallel analysis [Citation20] and a non-parametric bootstrap version of Kaiser’s rule [Citation21]. Both methods prescribed using six factors. The factor loadings were obtained using the Varimax rotation [Citation22]. The Variable Cutoff Method (VCM) was then applied to the factor loadings.
Results
Demographics and treatment technique values are summarized in . No significant associations were found between the investigated variables and the development of urgency syndrome. illustrates the 15 factor loadings onto the factor interpreted as urgency syndrome that were retained after the application of the Variable Cutoff Method. These 15 symptoms are associated and together comprise the urgency syndrome. Among them, ‘loose stools’, ‘need to repeat defecation within one hour’, ‘sudden defecation urgency requiring lavatory’, ‘degree of capacity to hold stools during urgency’ and ‘immediate need to defecate’ had the highest loadings.
Table 2. Demographic, clinical characteristics and treatment characteristics of survivors with or without ‘radiation-induced urgency syndrome’ after pelvic radiation therapy.
illustrates the mean and maximum non-corrected absorbed doses for the three OARs for patients with and without urgency syndrome. The p values were significant for the rectum and sigmoid colon both with respect to mean and maximum doses. The r value was very high (0.97) for the rectum, but was also high for the sigmoid colon (0.81) and small intestine (0.80).
Table 3. Mean and maximum absorbed doses for the three OARs, for patients with and without ‘radiation-induced urgency syndrome’ and correlation coefficients between mean and maximum absorbed doses.
The dose–volume response parameters using the Probit, RS, LKB and gEUD models for the rectum, sigmoid colon and small intestine are presented in . The calculated dose–response relations are illustrated in . Our results indicate that the sigmoid colon had the highest γ50 value (1.20, 1.25, and 1.27) for all three models and the highest area under the curve (AUC) (0.661 and 0.67) for the corrected mean doses () and the uncorrected doses (), respectively. For the rectum, sigmoid colon and the small intestine, the AIC value was lowest for the Probit model. However, the differences between the AIC values were small (Supplementary Table S3e). Dose–response relationships for all 530 cancer survivors (received EBRT and BT but had no stoma and had DVHs) are presented in the Supplementary Table S1e). These results indicate that sigmoid colon has the best fit for all models used among the studied OARs.
Figure 2. Dose–response curves of mean dose to the rectum, sigmoid colon and the small intestine using the (a) Probit model, (b) the Relative Seriality (RS) model, (c) the LKB model and (d) the mean dose to the sigmoid colon for Probit, the Relative Seriality (RS) and the LKB models. The black x show the survivors with the syndrome, while the gray x show the survivors without the syndrome.
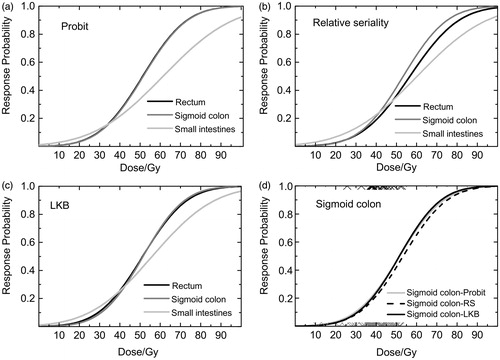
Table 4. The maximum likelihood estimates of the dose–response parameters for ‘radiation-induced urgency syndrome’ for the three OARs using Probit, RS, LKB and EUD models and the values for the AUC.
Discussion
Our results indicate that it is the dose to the sigmoid colon as well as to the rectum that determine the intensity of ‘radiation-induced urgency syndrome’. Both the sigmoid colon and rectum have strong dose–response relationships for this syndrome; both have steep dose–response curves (, ). Our results for the Probit, RS and LKB model indicate that the model used does not influence the result.
Among the studied OARs, the sigmoid colon had the steepest dose–response relationship, having the highest γ50 value. The sigmoid colon also had the highest AUC value (0.661). D50 could be used as a guideline for tissue tolerance dose and for the sigmoid colon it was 51.4 Gy. The estimated values for the volume effect parameters s, n and a indicate that the sigmoid colon has a serial behavior for urgency syndrome. However, the wide confidence intervals and the r value (0.81) adds a significant uncertainty to the estimated values of the volume parameters. The value of r means that the mean and maximum doses were correlated but not strongly linearly correlated. In the literature, there are not many studies that report dose–volume response relationships from gynecological cancer survivors and there are even fewer that report on having studied the sigmoid colon as an organ at risk. In the study by Fonteyne et al., the sigmoid colon irradiated with intermediate doses (S40S60) was suggested to be co-responsible for developing lower intestinal toxicity along with the anal sphincter and the rectum [Citation23]. There are also studies from our group [Citation9,Citation11] indicating that dose to the sigmoid colon is crucial for development of a self-assessed atomized symptom ‘defecation into clothing without forewarning’. In one of our previous studies [Citation9], we studied the dose–volume response relationship between the above symptom and anal-sphincter, rectum, sigmoid colon and small intestine and we found that the sigmoid colon had the steepest dose–volume response relationship (γ50 = 1.45, D50 = 56.9 Gy). This symptom is included in the urgency syndrome.
The rectum was also found to have a steep dose–response relationship for the syndrome (γ50 = 1.9, m = 0.47 and D50 = 51.3 Gy. We are not able to make suggestions regarding the volume effect of the rectum. The confidence intervals of the estimated volume effect parameter were very wide and the r value very high (0.97) (). The r value indicates that mean and max doses are linearly correlated. The small intestine seems to have a weak dose–response relationship (γ50 = 0.90, D50 = 61.4 Gy for Probit and AUC 0.63). We are unable to make any conclusion for the volume effect for the small intestine, because the confidence intervals for s (RS) were very wide and for n (LKB) parameter were not possible to be estimated. In the literature, there are few studies reporting dose–response relationships for the rectum after pelvic radiation therapy. Smeenk et al. [Citation24] reported that urgency and incontinence originate from both the anal wall and rectal wall, while frequency seemed mostly associated with rectal wall dysfunction. They also found that dose–effect relations differed between the described symptoms. That is in-line with our finding that there is dose–response relationship of rectum and the urgency syndrome, which includes urgency and frequency symptoms. On the other hand, we believe that the dose to the anal sphincter structure is not related with the syndrome. After radiotherapy for prostate cancer, disturbed (distal) colonic motility has been reported to contribute to anorectal symptoms and dysfunction [Citation25]. The latter report lends credence to the findings in this study that the dose to the sigmoid colon as well as the rectum contributes most to the urgency syndrome. Emami [Citation26] reported that high doses to the rectum are most important in determining the risk of rectal toxicity. Thor et al. [Citation8], reported LKB dose–volume response relationships for prostate cancer survivors, for rectum and defecation urgency, D50 = 83 Gy, m = 0.15, a = 100 and D50 = 68 Gy, m = 0.53, a = 3.00. Guliford et al. [Citation7], reported LKB parameters for rectum and rectal urgency D50 = 55.0 Gy, m = 0.48, n = 0.26.
The strength of our study is the access in high-quality data. Moreover, in our division we have developed clinometric methods to document patient-reported long-term symptoms [Citation27–30]. One limitation of our study results from the possible setup errors, which are expected to introduce a noise that dilute the calculated dose–effect relationships. Another limitation is that the rectum, sigmoid colon and small intestine are highly mobile organs and it is difficult to estimate the exact location of the organs during EBRT. In addition, the fact that the dose to different organs appear to be not very heterogeneous adds uncertainty to the estimated dose–response relationships. Additionally, dose–volume data for the small intestine was extracted for part of the organ and that would add uncertainty to the dose–volume response relations. There were also a few CT scans with 2 cm distance where delineation was difficult. In this study, the 85th percentile of the factor scores was used as a cutoff when classifying patients as suffering from or not suffering from urgency syndrome. Setting the cutoff value to 85th percentile allows us to avoid false positives in this classification while at the same time avoiding false negatives.
The α/β values that we found indicate that rectum and sigmoid colon are late responding tissues for urgency syndrome, while the small intestine responds early for this outcome. However, the confidence intervals of α/β values were very wide, which introduce a significant uncertainty in the calculated values. Our results are relevant for the development of the syndrome 3–12 years after radiotherapy. More information is needed before we can generalize them for the development of the syndrome before 3 years of follow-up.
Our results suggest that there are steep dose–response relations for the rectum and the sigmoid colon and ‘radiation-induced urgency syndrome’. The study provides evidence that separate delineation of the sigmoid colon and rectum maybe considered as being important and useful in reducing the incidence of the syndrome. No significant associations were found between demographic variables, treatment techniques and the development of ‘radiation-induced urgency syndrome’. All the models used yielded equal results indicating no preference for any specific model.
Eleftheria_Alevronta_et_al._Supplementary_files.zip
Download Zip (299.6 KB)Acknowledgments
We thank Dr. Paul Doliotis for his help with programing issues.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Steineck G, Skokic V, Sjöberg F, et al. Identifying radiation-induced survivorship syndromes affecting bowel health in a cohort of gynecological cancer survivors. PLoS One. 2017;12:e0171461.
- Steineck G, Schmidt H, Alevronta E, et al. Toward restored bowel health in rectal cancer survivors. Semin Radiat Oncol. 2016;26:236–250.
- Yeoh E, Sun WM, Russo A, et al. A retrospective study of the effects of pelvic irradiation for gynecological cancer on anorectal function. Int J Radiat Oncol Biol Phys. 1996;35:1003–1010.
- Ebert MA, Foo K, Haworth A, et al. Gastrointestinal dose-histogram effects in the context of dose-volume-constrained prostate radiation therapy: analysis of data from the RADAR prostate radiation therapy trial. Int J Radiat Oncol Biol Phys. 2015;1;91:595–603.
- Fiorino C, Valdagni R, Rancati T, et al. Dose-volume effects for normal tissues in external radiotherapy: pelvis. Radiother Oncol. 2009;93:153–167.
- Gulliford SL, Foo K, Morgan RC, et al. Dose-volume constraints to reduce rectal side effects from prostate radiotherapy: evidence from MRC RT01 Trial ISRCTN 47772397. Int J Radiat Oncol Biol Phys. 2010;76:747–754.
- Gulliford SL, Partridge M, Sydes MR, et al. Parameters for the Lyman Kutcher Burman (LKB) model of Normal Tissue Complication Probability (NTCP) for specific rectal complications observed in clinical practise. Radiother Oncol. 2012;102:347–351.
- Thor M, Olsson CE, Oh JH, et al. Relationships between dose to the gastro-intestinal tract and patient-reported symptom domains after radiotherapy for localized prostate cancer. Acta Oncol. 2015;54:1326–1334.
- Alevronta E, Lind H, Al-Abany M, et al. Dose-response relationships for an atomized symptom of fecal incontinence after gynecological radiotherapy. Acta Oncol. 2013;52:719–726.
- Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989;16:1623–1630.
- Lind H, Alevronta E, Steineck G, et al. Defecation into clothing without forewarning and mean radiation dose to bowel and anal-sphincter among gynecological cancer survivors. Acta Oncol. 2016;55:1285–1293.
- ICRU. Prescribing, recording, and reporting photon beam therapy. Bethesda: International Commission on Radiation Units and Measurements; 1993. (ICRU Report 50).
- Lind H, Waldenstrom AC, Dunberger G, et al. Late symptoms in long-term gynaecological cancer survivors after radiation therapy: a population-based cohort study. Br J Cancer. 2011;105:737–745.
- Gill PE, Murray W, Saunders MA, et al. User’s guide for NPSOL 5.0: A FORTRAN package for nonlinear programming. 1998. Technical Report SOL 86-6. 2001. Available from:https://web.stanford.edu/group/SOL/guides/npdoc.pdf
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–S19.
- Källman P, Ågren A, Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol. 1992;62:249–262.
- Niemierko A. Reporting and analyzing dose distributions: a concept of equivalent uniform dose. Med Phys. 1997;24:103–110.
- Niemierko AA. The generalized concept of equivalent uniform dose (EUD). Med Phys. 1999;26:1100 [abstract].
- Hall J.E. GJA, editor. Radiobiology for the radiobiologist. 6th ed. Phiadelphia: Lippincott Williams & Wilkins; 2006.
- Buja A, Eyuboglu N. Remarks on parallel analysis. Multivariate Behav Res. 1992;27:509–540.
- Zientek LR, Thompson B. Applying the bootstrap to the multivariate case: bootstrap component/factor analysis. Behav Res Methods. 2007;39:318–325.
- Kaiser H. The varimax criterion for analytic rotation in factor analysis. Psychometrika. 1958;23:187–200.
- Fonteyne V, De Neve W, Villeirs G, et al. Late radiotherapy-induced lower intestinal toxicity (RILIT) of intensity-modulated radiotherapy for prostate cancer: the need for adapting toxicity scales and the appearance of the sigmoid colon as co-responsible organ for lower intestinal toxicity. Radiother Oncol. 2007;84:156–163.
- Smeenk RJ, Hopman WP, Hoffmann AL, et al. Differences in radiation dosimetry and anorectal function testing imply that anorectal symptoms may arise from different anatomic substrates. Int J Radiat Oncol Biol Phys. 2012;82:145–152.
- Yeoh EK, Bartholomeusz DL, Holloway RH, et al. Disturbed colonic motility contributes to anorectal symptoms and dysfunction after radiotherapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2010;78:773–780.
- Emami B. Tolerance of normal tissue to therapeutic radiation. Radiother Oncol. 2013;1:25–48.
- Bergmark K, Åvall-Lundqvist E, Dickman PW, et al. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med. 1999;340:1383–1389.
- Kreicbergs U, Valdimarsdottir U, Onelov E, et al. Talking about death with children who have severe malignant disease. N Engl J Med. 2004;351:1175–1186.
- Steineck G, Bergmark K, Henningsohn L, et al. Symptom documentation in cancer survivors as a basis for therapy modifications. Acta Oncol. 2002;41:244–252.
- Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–796.
