Abstract
Background
The adaptation criteria for administration of stereotactic body radiotherapy (SBRT) to patients with lung cancer who previously underwent surgery and subsequently developed a second primary lung cancer (SPLC) or intra-parenchymal lung metastasis (IPLM) are controversial, unlike the criteria for repeat surgery. We aimed to evaluate the feasibility of SBRT for these patients. Factors associated with decreased respiratory function were also evaluated.
Material and methods
Sixty-nine patients with 89 lesions who underwent SBRT between 2008 and 2017 were analyzed. Of these, 29 were diagnosed with SPLC while the remaining 40 had IPLM. The distribution of histological types was as follows: squamous cell carcinoma (n = 13 lesions); adenocarcinoma (n = 25); non-small cell carcinoma (n = 1); unknown histological type (n = 49). The prescribed doses to the planning target volume (PTV) were 50 Gy in five fractions for 85 lesions and 60 Gy in 10 fractions for four lesions at PTV mean.
Results
Over a median follow-up period of 55 months, the 4-year overall survival and local control rates were 50.3% and 87.6%, respectively. Six patients experienced grade 2 radiation pneumonitis and one experienced grade 3. Two patients experienced grade 5 pulmonary fibrosis. Decreased respiratory function was observed in 10 patients (15.1%). On multivariate analysis, the presence of pulmonary disease before SBRT was the only statistically significant factor associated with decreased respiratory function.
Conclusions
SBRT is safe and feasible in patients with SPLC or IPLM previously treated surgically. Pre-existing pulmonary disease was a predictive factor for decreased respiratory function.
Introduction
A second primary lung cancer (SPLC) or intra-parenchymal lung metastasis (IPLM) is occasionally detected in patients during follow-up after undergoing radical surgery for lung cancer. The reported risk of developing an SPLC in patients who undergo resection of non-small cell lung carcinomas is approximately 1–2% per patient per year [Citation1]. Thus, long-term follow-up after the initial surgery is crucial to increase SPLC and IPLM detection rates [Citation2,Citation3]. There are significant differences in the treatment and prognosis of SPLC and IPLM; however, even if the diagnostic criteria for metachronous tumors devised by Martini and Melamed are consistent with SPLC in patients undergoing repeat surgery, some of these patients are deemed to have IPLM [Citation4]. Therefore, eligible patients with IPLM often undergo repeat surgery. In cases where the patient is unable to withstand surgery or refuses it, radiotherapy is often administered instead.
Stereotactic body radiotherapy (SBRT) for stage I non-small cell lung cancer or metastatic lung tumors less than 3 cm wide can achieve good local control (LC) and is associated with mild adverse events if a high radiation dose is concentrated directly onto the target [Citation5–8]. Therefore, if SPLCs or IPLMs are located in the lung field and are relatively small, SBRT is favored in lieu of further invasive treatment. The criteria for repeat surgery are well established, and include a post-surgical predicted forced expiratory volume in one second ≥800 mL and percent forced vital capacity (%VC) ≥ 40% [Citation9,Citation10]. Conversely, the criteria for conventional radiotherapy or SBRT have not yet been established. There are no reports adapting SBRT for the treatment of patients with SPLC or IPLM who were previously treated surgically; furthermore, exacerbating factors for decreased respiratory function have not been determined. Instead, the choice of treatment is at the discretion of radiation oncologists.
We therefore evaluated the feasibility of SBRT for SPLC and IPLM in patients previously treated surgically for lung cancer. We also investigated the factors related to decreased respiratory function.
Material and methods
Patient inclusion criteria
This study included patients treated with SBRT who previously underwent surgery for lung cancer and were found to have SPLC or IPLM on follow-up at our institution between September 2006 and November 2015. Written informed consent was obtained from all patients before undergoing treatment; our institutional review board approved this retrospective analysis (no. 16-18). All patients were evaluated by a multidisciplinary thoracic oncology group that comprised thoracic surgeons, pulmonologists and radiation oncologists.
The adapted criteria for SBRT to treat lung tumors at our institution are as follows: (1) no simultaneous regional lymph node metastases (i.e., to the hilar, mediastinal or supraclavicular lymph nodes) or any distant metastases, (2) performance status ≤3 and (3) tumors <5 cm in diameter and total number <3 lesions. There are no clear criteria at our institution with respect to respiratory function. Although the exclusion criteria covered patients lost to follow-up who had not experienced recurrence within 6 months after the completion of SBRT, no such patients were found in our cohort. Ultimately, we included 69 patients with 89 lesions; the characteristics of all patients and tumors are summarized in . The distribution of histological types was as follows: squamous cell carcinoma (n = 13 lesions), adenocarcinoma (n = 25), non-small cell carcinoma (n = 1) and unknown histological type (n = 49). Pulmonary diseases included chronic obstructive pulmonary disease (COPD), interstitial pneumonitis (IP; n = 2) and pulmonary aspergillosis (n = 1). All cases of COPD were emphysema.
Table 1. Patient characteristics.
Treatments
In April 2010, a new Novalis-Tx (Brain LAB AG, Feldkirchen, Germany) for SBRT was installed at our institution, the protocol for which was modified as a result. Protocols before and after the change are described below.
September 2006–April 2010
The patient was immobilized with a vacuum pillow, hip fixation board and Exacerbar (Brainlab AG, Feldkirchen, Germany). The target was defined as the gross tumor volume (GTV)=clinical target volume (CTV)=internal target volume (ITV). The ITV was determined according to the averaged computed tomography (CT) image acquired four times during normal breathing. The planning target volume (PTV) was defined as the ITV plus 5 mm in all directions. The beam energy was 4 and/or 10 MeV. Eight non-coplanar beams were used for treatment delivery. Irradiation was performed with a linear accelerator (CLINAC 21EX, Varian Medical Systems, Tokyo, Japan).
May 2010–November 2015
The patient was immobilized with a vacuum pillow and Exacerbar. The target was defined as GTV = CTV = ITV. The ITV was determined using four-dimensional CT during normal breathing; the mass on expiratory phase CT was defined as the ITV. The PTV was defined as the ITV plus 5 mm on the lower side and 3 mm on the remaining sides. The beam energy was 6 MeV. Eight non-coplanar beams were used for treatment delivery. A respiratory synchronization system using infrared markers was used for patients with stable respiration, synchronized with the expiratory phase. Irradiation was performed with a Novalis-Tx.
Dose regimens consisted of 50 Gy in five fractions for peripheral tumors and 60 Gy in 10 fractions for central tumors during both periods. The plan normalization method involved delivering 100% of the dose prescription at PTV mean. Details of all SBRT treatments are summarized in . Moreover, 10 patients underwent chemotherapy after SBRT, six of whom underwent molecular targeted therapy.
Table 2. Treatment details.
Definitions of SPLC and IPLM
Tumor histology was difficult to differentiate and was not always determined in SBRT patients for different reasons. We defined SPLC according to the following criteria: (1) lesions of a different histological type than the original, (2) lesions appearing >5 years since the initial surgery or (3) lesions with a ground glass nodule.
Follow-up
After SBRT, chest radiography was performed every 1–2 months and chest CT examinations were performed every 3–4 months. Magnetic resonance imaging of the brain and 18F-fluorodeoxyglucose positron emission tomography examinations were performed when assessment of the brain or of other extra-pulmonary metastases was required on the basis of clinical symptoms. Acute, sub-acute and chronic toxicity was evaluated using the National Cancer Institute-Common Toxicity Criteria Version 4.0.
We evaluated respiratory function using the British Medical Research Council (MRC) scale [Citation11] at the time of radiotherapy, and reevaluated patients at every post-treatment visit. Any decrease in the MRC scale by one grade or more was considered a clinically significant event.
Overall survival (OS) was calculated from the date of initial SBRT commencement to the date of death from any cause. LC was calculated from the date of initial SBRT commencement to the date of regrowth detection. Data of patients who experienced no events of interest were censored at the last follow-up date.
Primary endpoint and statistical analysis
The primary endpoint in this study was the feasibility of SBRT in patients previously treated with surgery, and we also wanted to identify predictors of decreased respiratory function.
The data were analyzed using SPSS software, version 24 (SPSS Inc., Chicago, IL, USA). OS and LC were evaluated using the Kaplan–Meier estimates and the log-rank test. Pearson’s χ2 test was used to assess the association between decreased respiratory function and different factors. Furthermore, on multivariate analysis, binomial logistic regression analysis was used. A p value of .05 or less was considered statistically significant; all statistical tests were two-sided.
Results
Advanced age and decreased respiratory function were the most common reasons for selecting SBRT instead of surgery; only two patients rejected surgery by their own discretion. The median follow-up time for all surviving patients was 55 months (range 3–105 months).
Survival outcomes
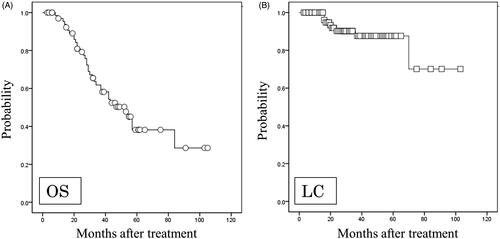
Thirty-three patients developed recurrences during the follow-up period; five experienced local recurrence only, nine experienced recurrences in other regions of the lung, one experienced local and regional lymph node recurrences, one experienced local and distant metastases, four experienced regional lymph node recurrences only, and 13 experienced distant metastases only. Twenty-three patients died of lung cancer, and 10 died of other causes (two of cerebral infarction, two of pulmonary fibrosis (PF), two of unknown causes, two of cardiac failure, one of laryngeal cancer, and one of infection). Only one patient was lost to follow-up at 37 months with no recorded recurrence. The OS rate for all patients was 50.3% at four years (). The four-year LC rates for all lesions were 87.6% (). No significant differences were found with respect to OS (p = .714) between patients with SPLC and those with IPLM.
Adverse events
Six patients developed grade 2 radiation pneumonitis (RP) and one developed grade 3 RP. Five patients developed a symptomatic rib fracture. A grade 1 pleural effusion was found in one patient, while a grade 2 skin reaction was reported in another. Conversely, two patients had a grade 5 PF and mild IP was suspected based on CT scans obtained before SBRT.
Evaluation of respiratory function
Ten patients (15.1%) had decreased respiratory function according to the MRC scale. Five patients had decreased respiratory function by ≤2 grades, and home oxygen therapy (HOT) was introduced for two patients. We evaluated the association between decreased respiratory function (based on the MRC scale) and different factors (). Univariate analysis revealed that the following factors were significantly associated with decreased respiratory function: a higher Brinkman index (p = .046), the presence of pulmonary disease before SBRT (p = .024), multiple SBRT treatments (p = .039) and chemotherapy after SBRT (p = .015). Next, before performing multivariate analysis, we analyzed the correlation between the Brinkman index and COPD. As expected, a strong correlation was identified (p < .001). Therefore, multivariate analysis was performed using the above three factors (except for the Brinkman index) and the presence of pulmonary disease before SBRT was found to be the only statistically significant factor associated with decreased respiratory function (p = .028). The details of the 10 patients with decreased respiratory function are shown in .
Table 3. Analysis of clinical and dosimetric variables associated with decreased respiratory function (n = 69).
Table 4. Summary of patients with decreased respiratory function after stereotactic body radiotherapy.
Grade 5 PF
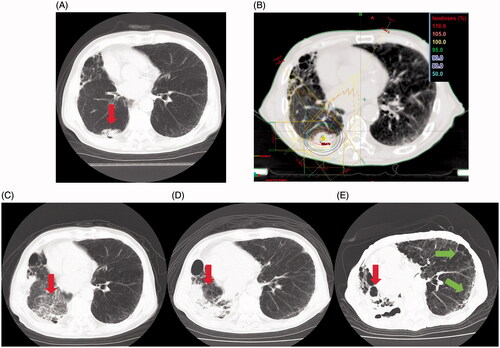
The CT findings of a 78-year-old man, one of two patients with grade 5 PF, are shown in . The 3.5-cm mass was located in the right lower lobe (); squamous cell carcinoma with cT2N0M0 stage IB was diagnosed. The patient had a medical history of pulmonary emphysema and right lower lobe resection for lung cancer five years previously, and we detected signs of mild IP in the contralateral lung on diagnostic imaging. Before SBRT, his respiratory function was assessed as grade 1. The ITV and PTV for SBRT were 19.2 mL and 42.6 mL, respectively, and dose regimens were 50 Gy in five fractions using eight non-coplanar static beams (). At 3 months after SBRT completion, a frosted glassy shadow emerged, and the tumor shrank (). At 13 months after SBRT, the lung volume had severely contracted in the region of the irradiated field (). At 28 months after SBRT, an IP-like pattern emerged bilaterally in the pulmonary region outside the irradiated field, and honeycomb lung in the peripheral area was observed (). HOT was introduced; however, the patient died of respiratory insufficiency at 29 months. The remaining patient was an 85-year-old man with a 3.2-cm mass located in the left lower lobe diagnosed as IPLM. The patient had previously undergone surgery on three occasions; therefore, the %VC considerably decreased to 60.7%. Mild IP and emphysema in the contralateral lung were detected via CT. The patient underwent SBRT; dose regimens were 50 Gy in five fractions using eight non-coplanar static beams. At 3 months after SBRT completion, grade 3 RP developed in the irradiated field and the patient developed concurrent mediastinal emphysema. Although HOT was introduced due to deterioration of respiration and worsening of daily activity, the patient died of respiratory insufficiency at 14 months post-SBRT.
Figure 2. Disease course of the patient with grade 5 pulmonary fibrosis. (A) Computed tomography (CT) before stereotactic body radiotherapy (SBRT) revealed a mass in the right lower lobe (red arrow). (B) Treatment planning for SBRT (50 Gy in five fractions). (C) CT findings 3 months after SBRT revealed that the tumor decreased in size (red arrow) and a frosted glassy shadow appeared. (D) CT findings 1 year and 1 month after SBRT; the lung volume had severely contracted in the region of the irradiated field (red arrow). (E) CT findings 2 years and 4 months after SBRT. An interstitial pneumonitis-like pattern was observed bilaterally in the pulmonary region outside the irradiated field (red arrows), and honeycomb lung in the peripheral area was observed (green arrows).
Discussion
The adaptation of SBRT for patients previously treated with surgery has not been discussed before. In this study, data analysis revealed that the presence of pulmonary disease before SBRT was significantly associated with decreased respiratory function.
There are few studies of SBRT in patients previously treated with surgery [Citation12–15]. Takeda et al. originally analyzed the outcomes of SBRT in 23 patients with isolated post-surgical local recurrences (IPSLRs), including 21 patients with bronchial stump or staple line recurrences and two patients with chest wall recurrences. Three patients suffered from grade 3, 4 and 5 RP [Citation12]. The investigators concluded that SBRT for IPSLR achieved high LC with limited toxicity. Nishiyama et al. subsequently investigated 41 medically inoperable patients who underwent SBRT for second pulmonary nodules arising from different types of cancer, and reported that grade 2 RP toxicity occurred in five patients and one died of grade 5 RP [Citation13]. Two further studies by Chang et al. and Creach et al. assessed SBRT for multiple primary lung cancers, including patients who underwent surgery as the initial treatment [Citation14,Citation15]. Creach et al. reported no grade ≥3 toxicity; however, Chang et al. reported a 3% incidence of grade ≥3 RP, grade 3 chest wall pain, and grade 3 skin toxicity. The results of second resections for SPLC and IPLM reported by the thoracic surgery team at our institution are of interest, with Yukiue et al. reporting OS rates at two and five years of 87.8% and 62.9%, respectively [Citation16]. Of note, nine patients (23%) had postoperative complications and one (2.6%) died during surgery. Studies indicate that the general OS rates after repeat surgery for SPLC and IPLM are 26–56.5% at 5 years [Citation3,Citation17–21]. As many patients in our analysis were originally considered ineligible for surgery, we regard our results as promising in terms of LC and toxicity. However, the results of SBRT are not necessarily comparable to those of surgery, where a pathological specimen can be obtained to verify that the underlying disease is lung cancer. A histological diagnosis is not always determined in patients undergoing radiotherapy because CT-guided lung biopsies or endobronchial ultrasound-guided transbronchial needle aspiration may not be suitable for patients who have undergone lung resection.
Ten patients (15.1%) in this study had decreased respiratory function and, on multivariate analysis, the presence of pulmonary disease before SBRT was significantly associated with decreased respiratory function. Mentzer and Swanson reported that 20% of patients with COPD had decreased, inoperable respiratory function [Citation22]. Therefore, SBRT may markedly decrease respiratory function in patients with background pulmonary disease, such as COPD. Moreover, Kojima et al. reported that age and smoking (as measured by the Brinkman index) were strong risk factors for COPD [Citation23]. The significant association between the Brinkman index and decreased respiratory function may indicate the potential existence of COPD, and the association between the Brinkman index and COPD was confirmed in our study. Therefore, it is important to take into consideration the presence of COPD or of a high Brinkman index before delivery of SBRT.
Two patients (2.8%) with grade 5 PF were included in this study and we considered this rate to be acceptable. The two patients with grade 5 PF were diagnosed with mild IP on diagnostic imaging. Ozawa et al. investigated the clinical characteristics and predictive factors for developing acute extended RP with a focus on the presence and radiological characteristics of preexisting IP, and concluded that the presence of preexisting IP on the CT scan before radiotherapy was a significant predictive factor (odds ratio = 22.6; 95% confidence interval: 5.29–155; p < .001) [Citation24]. Lee et al. reported that the interstitial changes in the pre-RT chest CT scan (p = .009), mean lung dose (p < .001), and the percentage of lung volume receiving over 30 Gy (V30) (p < .001) significantly predicted RP on multivariate analysis. Therefore, despite the comparatively small field and minimal irradiation volume of SBRT, clinicians should consider the possibility of exacerbated IP [Citation25].
The association between the irradiated lung volume and decreased respiratory function should be further explored; however, in cases of multiple irradiations, it is difficult to precisely determine the irradiated lung volume and, therefore, the amount of radiation resulting in decreased respiratory function. Tsujino et al. examined the association between the percentage of lung volume receiving more than 20 Gy (V20) and RP; V20 is currently one of the most widespread indexes used by radiation oncologists for devising treatment plans [Citation26]. In an effort to develop a more accurate prediction model for severe RP, Tsujino et al. suggested that scoring factors, such as V20, lung volume spared from a 5 Gy dose (VS5), age, and PF on baseline CT, may improve the prediction of severe RP [Citation27]. Therefore, clearer indexes, such as V20, V30, VS5, or lung mean dose should be investigated for their suitability as predictive factors for decreased respiratory function under well-designed conditions.
In this study, we did not observe significant differences in the prognosis between patients with SPLC and IPLM, although the latter are presumed to have worse prognoses. We postulate that our grouping criteria were imperfect, and that some patients with SPLC were included in the IPLM group. However, accurate categorization based on image findings or tissue analysis may not be possible. It is difficult to distinguish SPLC from IPLM even surgically, despite the criteria developed by Martini or the American College of Chest Physicians [Citation4,Citation28]. Conversely, some studies of repeat surgery reported that patients with stage I SPLC had significantly better survival rates compared to patients with higher stage diseases [Citation17,Citation18]. Therefore, SPLC-targeting treatments should also be considered for isolated lung masses in SBRT.
The following limitations of this study should be considered: the retrospective nature, relatively small sample size, and single-center experience. Other limitations included the fact that many patients with known histological types were included, which could have created a bias.
In conclusion, SBRT is safe and feasible against SPLC or IPLM in patients previously treated with surgery. However, the presence of pulmonary disease can significantly affect respiratory function. Going forward, a prospective study is warranted to obtain a larger sample size and better identify significant predictive factors for decreased respiratory function in patients previously treated with surgery.
Ethics approval and consent to participate
Written informed consent was obtained from all patients prior to administering treatment; the Seirei Mikatahara General Hospital review board approved this retrospective study (no. 16-18).
Acknowledgments
We thank Editage (www.editage.jp) for English language editing.
Availability of data and materials
Datasets supporting the conclusions of this study are included in the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90:1335–1345.
- van Bodegom PC, Wagenaar SS, Corrin B, et al. Second primary lung cancer: importance of long term follow up. Thorax. 1989;44:788–793.
- Aziz TM, Saad RA, Glasser J, et al. The management of second primary lung cancers. A single centre experience in 15 years. Eur J Cardiothorac Surg. 2002;21:527–533.
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70:606–612.
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637.
- Stephans KL, Woody NM, Reddy CA, et al. Tumor control and toxicity for common stereotactic body radiation therapy dose-fractionation regimens in stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2018;100:462–469.
- Mitsuyoshi T, Matsuo Y, Shintani T, et al. Pilot study of the safety and efficacy of dose escalation in stereotactic body radiotherapy for peripheral lung tumors. Clin Lung Cancer. 2018;19:e287–e296.
- Baine MJ, Verma V, Schonewolf CA, et al. Histology significantly affects recurrence and survival following SBRT for early stage non-small cell lung cancer. Lung Cancer. 2018;118:20–26.
- Olsen GN, Block AJ, Tobias JA. Prediction of postpneumonectomy pulmonary function using quantitative macroaggregate lung scanning. Chest. 1974;66:13–16.
- Nakahara K, Monden Y, Ohno K, et al. 1985: a method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. 1992 update. Ann Thorac Surg. 1992;54:1016–1017.
- National Collaborating Centre for Chronic Conditions. Chronic obstructive pulmonary disease. National clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl.1):1–232.
- Takeda A, Sanuki N, Eriguchi T, et al. Salvage stereotactic ablative irradiation for isolated postsurgical local recurrence of lung cancer. Ann Thorac Surg. 2013;96:1776–1782.
- Nishiyama K, Kodama K, Teshima T, et al. Stereotactic body radiotherapy for second pulmonary nodules after operation for an initial lung cancer. Jpn J Clin Oncol. 2015;45:947–952.
- Chang JY, Liu LH, Zhu Z, et al. Stereotactic ablative radiotherapy: a potentially curable approach to early stage multiple primary lung cancer. Cancer. 2013;119:3402–3410.
- Creach KM, Bradley JD, Mahasittiwat P, et al. Stereotactic body radiation therapy in the treatment of multiple primary lung cancers. Radiother Oncol. 2012;104:19–22.
- Yukiue H, Tanahashi M, Haneda H, et al. [Surgical treatment for recurrent and second primary lung cancer]. Kyobu Geka. 2010;63:944–949 (Japanese).
- van Rens MT, Zanen P, de la Rivière AB, et al. Survival after resection of metachronous non-small cell lung cancer in 127 patients. Ann Thorac Surg. 2001;71:309–313.
- Battafarano RJ, Force SD, Meyers BF, et al. Benefits of resection for metachronous lung cancer. J Thorac Cardiovasc Surg. 2004;127:836–842.
- De Leyn P, Moons J, Vansteenkiste J, et al. Survival after resection of synchronous bilateral lung cancer. Eur J Cardiothorac Surg. 2008;34:1215–1222.
- Kocaturk CI, Gunluoglu MZ, Cansever L, et al. Survival and prognostic factors in surgically resected synchronous multiple primary lung cancers. Eur J Cardiothorac Surg. 2011;39:160–166.
- Koezuka S, Hata Y, Otsuka H, et al. Metachronous second primary lung cancer surgically treated five years or more after the initial surgery. Mol Clin Oncol. 2015;3:1025–1028.
- Mentzer SJ, Swanson SJ. Treatment of patients with lung cancer and severe emphysema. Chest. 1999;116(Suppl.6):477S–479S.
- Kojima S, Sakakibara H, Motani S, et al. Effects of smoking and age on chronic obstructive pulmonary disease in Japan. J Epidemiol. 2015;15:113–117.
- Ozawa Y, Abe T, Omae M, et al. Impact of preexisting interstitial lung disease on acute, extensive radiation pneumonitis: retrospective analysis of patients with lung cancer. PLoS One. 2015;10:e0140437.
- Lee YH, Kim YS, Lee SN, et al. Interstitial lung change in pre-radiation therapy computed tomography is a risk factor for severe radiation pneumonitis. Cancer Res Treat. 2015;47:676–686.
- Tsujino K, Hirota S, Kotani Y, et al. Radiation pneumonitis following concurrent accelerated hyperfractionated radiotherapy and chemotherapy for limited-stage small-cell lung cancer: dose-volume histogram analysis and comparison with conventional chemoradiation. Int J Radiat Oncol Biol Phys. 2006;64:1100–1105.
- Tsujino K, Hashimoto T, Shimada T, et al. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9:983–990.
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2003;143(Suppl.5):e369S–e399S.